Article Contents
| Clin Exp Pediatr > Volume 67(9); 2024 |
|
Abstract
Bacterial bloodstream infections (BSI) are the leading cause of mortality and morbidity in pediatric solid organ transplant recipients. This systematic review aimed to pool global data from leading transplant institutions and identify the overall incidence, risk factors, and causative organisms of BSI in pediatric liver transplant recipients. A systematic review of the PubMed and OVID databases was conducted from 2000 to 2022. The initial search yielded 252 unique articles, which were independently reviewed by 2 authors. Articles that reported pediatric-specific data on BSI in isolated liver transplant patients were included, including the incidence of BSI, isolated organisms, and involved risk factors involved. This systematic review was registered with PROSPERO (ID: CRD42023403206). Fourteen articles from the United States, France, Iran, Japan, Korea, South Africa, Thailand, and Turkey were included. A total of 4,812 liver transplants were included in the final analysis. The mean patient age was 25 months (age range, 0–18 years), and 50.9% were male. The overall incidence of BSI was 23.5% (range, 14.7%–55%). The most commonly reported organisms were Staphylococcus epidermidis, Enterococcus, Klebsiella spp., and Escherichia coli. Among the risk factors studied, postope rative biliary complications, a medical history of biliary atresia, and younger age were the risk factors most commonly associated with BSI. Bacterial BSI after pediatric liver transplantation occur at a high incidence, with a unique organism profile notable for a higher percentage of gram-negative organisms. Further studies are required to de-termine the most appropriate prophylactic and empirical antibiotic management strategies for this population.
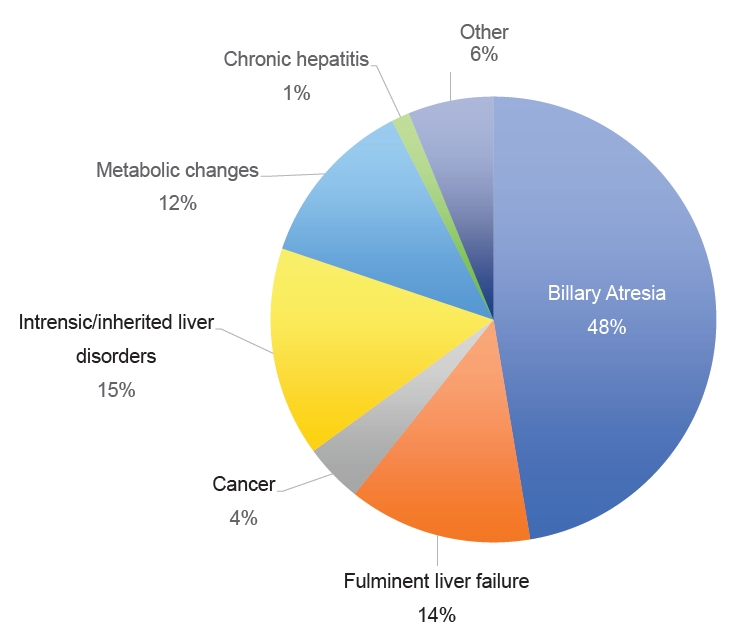
Liver transplantation (LT) is an effective and life-saving procedure for patients with end-stage liver disease. According to the United Network of Organ Sharing, 5,167 liver transplants were performed through July 2023 in the United States (US) alone, a dramatic increase over the past several years [1]. The Society of Pediatric Liver Transplantation reported that biliary atresia (38.5%) is the most common indication for LT in children in the US, followed by metabolic diseases, tumors, and fulminant liver failure [2].
The Centers for Disease Control and Prevention defines bloodstream infections (BSI) as blood cultures growing a recognized non-commensal bacterial or fungal organism not related to an infection at another site or the presence of at least one clinical sign (fever, chills, or hypotension) alongside 2 separate blood cultures growing the same organism [3].
Despite rapid advances in surgical techniques, antibiotic prophylaxis, immunosuppressive regimens, and infection control protocols, BSI remains an important cause of morbidity and mortality in pediatric LT recipients, with reported incidences of 38%–65% [4-6]. This could be attributed to frequent and prolonged hospitalizations along with the need for indwelling catheters, such as central venous catheters [7]. Moreover, the use of immunosuppressive medications has the dual effect of mitigating rejection rates while increasing the risk of infection [7].
Considering the heavy burden of BSI, understanding the spectrum of involved organisms is essential for choosing prophylactic and empirical antimicrobials, especially in the context of the global threat of antimicrobial resistance. The causative bacterial organisms vary among different patient subgroups based on their underlying morbidities, and pediatric LT recipients present unique risk factors for BSI. In addition, the transplant institutions included in this systematic review selected different antimicrobial regimens based on the rate of BSI and specific organisms encountered at each center. Although bacterial pathogens are the most common cause of BSI [8], fungal organisms have been reported to occur at rates of 5%–42% in LT recipients [9,10], with a mortality rate as high as 80% [11].
To the best of our knowledge, there have been no systematic reviews on BSI post-LT in an evidently vulnerable pediatric population. We performed a systematic review of published studies to determine the incidence, organisms involved, and risk factors for BSI in pediatric LT recipients.
A systematic review was performed according to the 2015 Preferred Reporting Items for Systematic Reviews on Meta-Analyses 2015 guidelines [12]. Two reviewers (MS and RH) independently identified relevant studies through an electronic search of the PubMed and OVID databases from inception to May 2023. The following search terms and keywords were used: BSI, sepsis, liver transplant, and LT. This systematic review was registered in the International Prospective Register of Systematic Reviews (No. CRD42023403206).
Two reviewers (MS and RH) independently assessed study eligibility based on title, abstract, and full-text report reviews. Eligible studies included information about the prevalence, risk factors, and involved organisms in BSI in pediatric LT recipients (0–21 years of age). The exclusion criteria were as follows: (1) pediatric cases not reported separately in studies including adults; (2) duplicate publications; (3) studies on patients with multivisceral transplantation; and (4) reviews, editorials, letters, and nonhuman studies. Only studies published in English were included in this systematic review.
Two investigators (MS and RH) independently extracted the data (prevalence, organisms, risk factors reported, demographics, indications for LT, and antimicrobial prophylactic medications used) using a standardized data abstraction form. Study methodological quality was assessed systematically using the Newcastle-Ottawa Scale for Observational Studies.
The initial database search identified 276 studies, of which 24 were duplicates and 3 were not retrievable and, therefore, excluded. Following the screening of titles and abstracts against the inclusion and exclusion criteria, 17 full-text reviews were performed, of which 3 were excluded. Finally, 14 full-text studies were included in this systematic review (Table 1) [5,13-25]. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram for the study search and selection process is displayed in Fig. 1.
In this review, 13 retrospective cohort studies and one case control study were included for a total of 14 studies from France, Iran, Japan, Korea, South Africa, Thailand, Turkey, and the US. The extracted data included the BSI rates (Table 1), organisms identified (Table 2) [5,13,16,18-20,22,23], prophylactic antibiotics used (Table 3) [5,13,15-17,19,20,22-24], and risk factors (Table 4) [13,19-21,23]. The patient population comprised 50.9% males (n=1,283), with a mean age of 25 months. A total of 4,812 LT were performed, 68% (n=1,481) of which were from living donors. The most common indications for LT were biliary atresia, inherited or intrinsic liver problems, and metabolic disorders (Fig. 2).
The incidence of BSI in the combined population was 23.5% (range, 14.7%–55%)(Fig. 3).
Considering the different institutes from across the globe included in this review, there were differences in the choice of perioperative prophylactic antibiotics and their doses. The most common prophylactic antibacterial agents used were ampicillin, ampicillin-sulbactam, piperacillin-tazobactam, and cephalosporins. Regarding antifungals, most institutions started fluconazole postoperatively, with a few notable exceptions, such as micafungin in the South African study reported by Duncan et al. [20] (Table 3).
The immunosuppressants tacrolimus and methylprednisolone were administered universally during the induction phase. Other agents with different efficacies include basiliximab, mycophenolate mofetil, and azathioprine (Table 3).
Although many studies have reported the risk factors for infections post-LT, only those listed in Table 4 specifically detailed the risk factors for BSI. Risk factors can be divided into 3 categories: preoperative (e.g., age, biliary atresia as the underlying indication, Pediatric End-Stage Liver Disease/Model for End-Stage Liver Disease [PELD/MELD] score, weight/height/growth failure, colonization with multidrug-resistant organisms, use of a liver support system), intraoperative (e.g., operative time, biliary and portal vein complications, blood volume lost and transfused, cold ischemia time, need for reoperation, ABO incompatibility), and postoperative (e.g., central catheter time, length of stay, and histological rejection).
Here we conducted a systematic review to examine the incidence, risk factors, and causative organisms of BSI among pediatric patients following LT worldwide. Our key findings were as follows:(1)the global incidence of BSI in LT patients was 24.61%; (2) the most common organisms were S. epidermidis, Enterococcus spp., and Klebsiella spp.; and (3) the most common risk factors identified were younger age at the time of LT, volume of blood loss, and medical history of biliary atresia.
Indications for LT in the pediatric population (Fig. 2) differ from those in adults. As highlighted in a review by Graziadei et al. [26] the most common indications for LT in adults include nonalcoholic steatohepatitis, alcoholic liver cirrhosis, and chronic hepatitis C. The underlying etiology plays a role in the prognosis of LT, as shown by Kim et al. [13] and Duncan et al. [20], which further emphasizes the importance of studying pediatric populations independently.
This review demonstrated that the incidence of BSI varies greatly among participating transplant centers (Fig. 3), which may be due to specific patient care-related factors (i.e., infection control measures, antimicrobial prophylaxis used) or to study-related factors (i.e., sample size, selection, and other potential biases). For example, while BSI prevalence was the highest in Turkey, it is important to note the small population in the Turkish study of 34 LT recipients; therefore, this may not reflect an accurate measure.
Other potential explanations for this variation include the use of different antimicrobial prophylactic regimens. France had the second highest incidence of BSI among the analyzed studies. Interestingly, the center in France relied on cefoxitin monotherapy from 2009 to 2011, which did not cover Enterococcus spp. and Pseudomonas spp., both of which are frequently isolated organisms.Additionally, donor population plays an important role in the risk of BSI. It is noteworthy that the study from Japan involved 97%–100% living donors, which is classically associated with fewer postoperative complications and better outcomes. The reported rates must be interpreted in conjunction with the follow-up period (Table 1). Iran had the lowest incidence. However, this finding was likely confounded by the short follow-up period (duration of hospital stay alone).
Outlining the spectrum of bacterial and fungal organisms involved in BSI is essential for the choice of prophylactic and empirical antimicrobials, especially in specialized populations such as pediatric LT recipients. The high prevalence of S. epidermidis and other gram-positive organisms is consistent with that in the general pediatric population [26].
However, gram-negative organisms occurred in BSI at a higher rate in LT recipients than in the general pediatric population. Kolesnichenko et al. [27] noted that the ratio of gram-positive to gram-negative organisms involved in BSI in the general pediatric population was 86%:11.6%, whereas our review found a ratio of 59.5%:40.5%.
Although the variable antibiotic regimens between centers (Table 3) could be due to local antibiograms and different resistance patterns, this finding emphasizes the need for empirical gram-negative coverage when choosing antimicrobials. Future studies are needed to examine whether there is a correlation between the use of certain antimicrobials and the incidence of BSI.
Identifying pre- and postoperative risk factors for BSI can potentially lead to stricter monitoring, with a lower threshold for collecting blood cultures and starting empiric antimicrobials in vulnerable groups. Many risk factors are shared between adult and pediatric LT recipients, including male sex, PELD/MELD score, operative time, blood loss, and ABO incompatibility [28]. However, some risk factors are unique to pediatric patients, such as biliary atresia as the underlying cause, younger age, and the graft-recipient body weight ratio.
A multicenter retrospective study of adult LT patients published in 2016 highlighted 2 important differences compared to our pediatric population: (1) the incidence of BSI was 29.4% (vs. the 24.6% rate found in our study in the pediatric population); (2) notably, an almost equal distribution of gram-positive (51%) and gram-negative (49%) organisms (vs. in the pediatric population, gram-positive organisms were more predominant at 59.5%). Another valuable observation was that older age was a significant risk factor in adults (vs. younger age in the pediatric population), suggesting that age extremes are a risk factor for BSI [29].
The limitations of our systematic review include the following: (1) different follow-up periods were used in the various selected articles, ranging from a few months to multiple years, suggesting that some late-onset BSI cases may have been overlooked; (2) multiple articles highlighted the risk factors for infection after pediatric LT without any BSI-specific risk factors, indicating that the information was not reported; and (3) certain populations, specifically in the US, were more fully represented (55% of the total population in our study) due to the higher research output.
In conclusion, bacterial BSI after pediatric LT has a high incidence, with a unique profile notable for a higher percentage of gram-negative organisms. Further studies are required to determine the most appropriate prophylactic and empirical antibiotic management strategies for this population.
Acknowledgments
The authors would like to thank Medstar Health, Georgetown University, Global Remote Research Scholars Program and Al Jalila Childrens’ Hospital for their continuous support.
Fig. 1.
PRISMA (Preferred Reporting Items for Systematic Reviews on Meta- Analyses) flow diagram summarizing the study screening process.
Fig. 3.
Incidence of bloodstream infections (BSI) after pediatric liver transplantation among included studies.
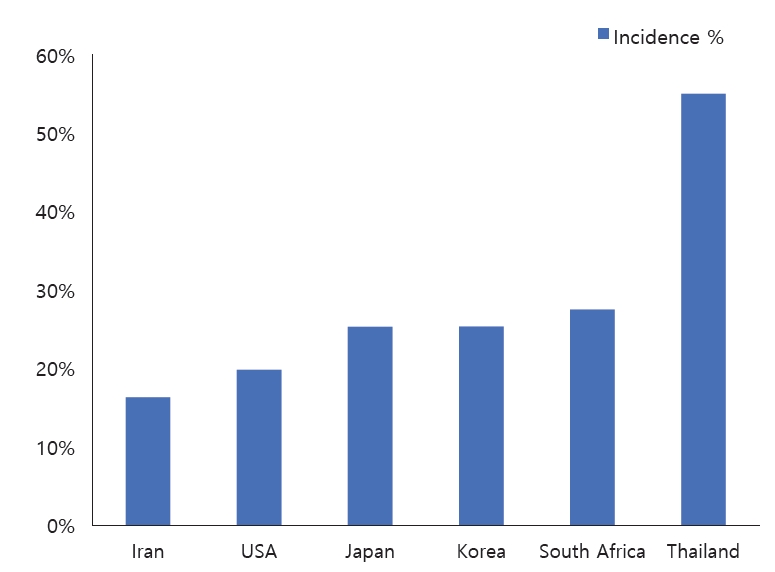
Fig. 4.
Causative organisms of bloodstream infections among pediatric liver transplantation patients.
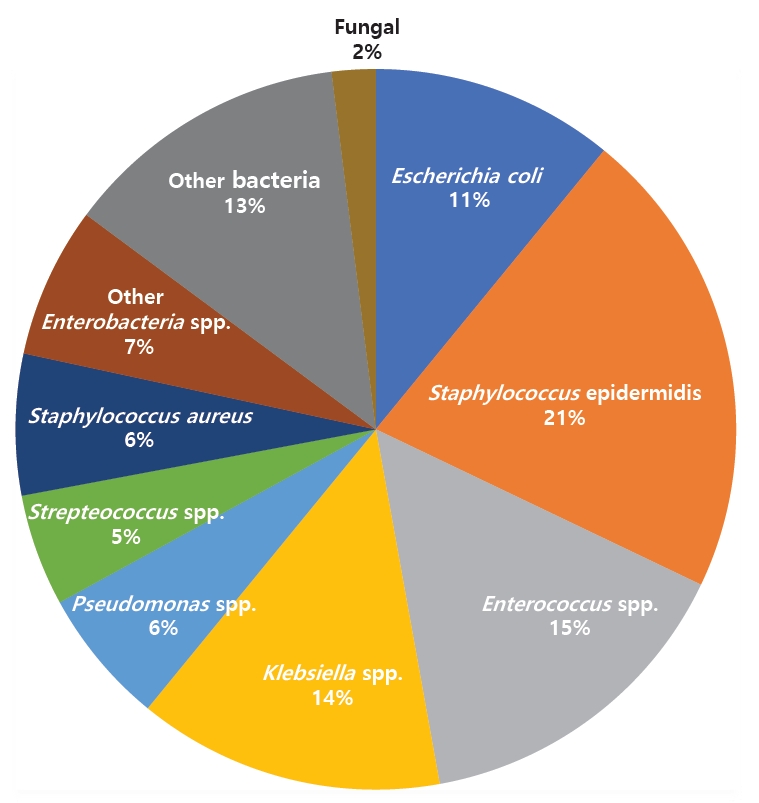
Table 1.
Detailed breakdown of included studies, patient demographics, incidence of bloodstream infections (BSI), and follow-up period
| No. | Study | Title | Country | Study design | Study period | Sex | Age (mo) | No. of liver transplants | No. of BSI | Incidence | Living liver trans plant % | Follow-up period |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Kim et al., [13] 2022 | Assessment of pathogens and risk factors associated with bloodstream infection in the year after pediatric liver transplantation | Korea | Retrospective cohort | 1994–2020 | 53% | 19 | 378 | 106 | 28% | 76% | 12 months |
| 2 | Sintusek et al., [14] 2021 | Immunization status and hospitalization for vaccine-preventable and non-vaccine-preventable infections in liver-transplanted children | Thailand | Retrospective cohort | 2004–2018 | 53.2% F | 30 | 77 | 32 | 41.50% | N/A | 5 Years, detailed breakdown available |
| 3 | Béranger et al., [15] 2020 | Early Bacterial Infections After Pediatric Liver Transplantation in the Era of Multidrug-resistant Bacteria-Nine-year Single-center Retrospective Experience | France | Retrospective cohort | 2009–2017 | 48% | 16 | 142 | 27 | 19 | N/A | <1 Month |
| 4 | Balcı Sezer et al., [16] 2020 | Prevelance of infections in infants within the first 6 months of liver transplant | Turkey | Retrospective cohort | 2005–2018 | 41% F | 8 | 34 | 19 | 55% | N/A | 6 Months |
| 5 | Phichaphop et al., [17] 2020 | High prevalence of multidrug-resistant gram-negative bacterial infection following pediatric liver transplantation | Thailand | Retrospective cohort | 2010–2019 | 56% F | 20 | 118 | 32 | 27.12 | 95.80% | 3 Months |
| 6 | Alcamo et al., [18] 2020 | Severe Sepsis in Pediatric Liver Transplant Patients: The Emergence of Multidrug-Resistant Organisms | USA | Retrospective cohort | 2010–2016 | 52.8% | 24 | 173 | 27 | 15.6 | 33%* | 6 Months |
| 7 | Dohna Schwake et al., [19] 2020 | Bacterial infections in children after liver transplantation: A single-center surveillance study of 345 consecutive transplantations | France | Retrospective cohort | 2006–2015 | 51.8% | 52 | 345 | 127 | 36.8 | 13% | <1 Month |
| 8 | Duncan et al., [20] 2019 | Blood stream infections in children in the first year after liver transplantation at Wits Donald Gordon Medical Centre, South Africa | South Africa | Retrospective cohort | 2005–2014 | 61% | 49 | 69 | 19 | 27.5 | 23% | 12 Months |
| 9 | Shoji et al., [21] 2019 | Risk Factors for Bloodstream Infection After Living-donor Liver Transplantation in Children | Japan | Retrospective cohort | 2005–2013 | 59.6% | 14 | 210 | 53 | 25.23 | 100% | Unspecified |
| 10 | Pouladfar et al., [22] 2019 | Bacterial infections in pediatric patients during early post liver transplant period: A prospective study in Iran | Iran | Case control | 2014–2015 | 51.1% | 7 | 97 | 16 | 16.5 | N/A | Hospital stay |
| 11 | Furuichi et al., [23] 2018 | Characteristics and Risk Factors of Late-onset Bloodstream Infection Beyond 6 Months After Liver Transplantation in Children | Japan | Retrospective cohort | 2005–2016 | 57% | 14 | 392 | 155 | 39.54 | 97% | >6 Months, median follow-up 1,571 |
| 12 | Shepherd et al., [5] 2013 | Risk Factors for Rejection and Infection in Pediatric Liver Transplantation | USA | Retrospective cohort | 1996–2006 | N/A | N/A | 2,291 | 461 | 20.12 | N/A | Unspecified |
| 13 | Nafady-Hego et al., [24] 2011 | Pattern of bacterial and fungal infections in the first 3 months after pediatric living donor liver transplantation: an 11-year single-center experience | Japan | Retrospective cohort | 1998–2009 | 56% | 47 | 391 | 43 | 10% | 100% | 3 Months |
| 14 | Kim et al., [25] 2010 | Infections after Living Donor Liver Transplantation in Children | Korea | Retrospective cohort | 1994–2004 | 60% | 22 | 95 | 14 | 14.7 | 100% | Unspecified |
Table 2.
Organisms isolated in the bloodstream reported by the included studies
| Study | E. coli (n) | Enterobacter (n) | Enterococcus sp (n) | Klebsiella spp. (n) | Pseudomonas (n) | S. epidermidis (n) | Staph (n) | Strep (n) | Other (n) | Fungal (n) |
|---|---|---|---|---|---|---|---|---|---|---|
| Kim et al., [13] 2022 | 6 | 9 | 21 | 11 | 3 | 67 | 5 | 17 | 35 | 2 |
| Balcı Sezer et al., [16] 2020 | 0 | 0 | 4 | 4 | 0 | 4 | 0 | 0 | 7 | 0 |
| Alcamo et al., [18] 2020 | 3 | 1 | 9 | 2 | 0 | 0 | 1 | 0 | 4 | 0 |
| Dohna Schwake et al., [19] 2020 | 17 | 6 | 11 | 8 | 17 | 12 | 6 | 3 | 3 | 0 |
| Duncan et al., [20] 2019 | 4 | 2 | 7 | 14 | 2 | 0 | 0 | 0 | 0 | 3 |
| Pouladfar et al., [22] 2019 | 4 | 0 | 9 | 0 | 0 | 2 | 0 | 0 | 3 | 0 |
| Furuichi et al., [23] 2018 | 10 | 4 | 3 | 7 | 6 | 3 | 0 | 3 | 7 | 0 |
| Shepherd et al., [5] 2013 | 6 | 9 | 5 | 17 | 0 | 9 | 17 | 0 | 0 | 4 |
| Total | 50 | 31 | 69 | 63 | 28 | 97 | 29 | 23 | 59 | 9 |
Table 3.
Antimicrobial prophylaxis and immunosuppressants used in the included studies
| Study | Country | Antimicrobial prophylaxis involved | Immunosuppressants |
|---|---|---|---|
| Kim et al., [13] 2022 | Korea | Ampicillin plus sulbactam (150 mg/kg per day) and cefotaxime (100 mg/kg per day) intravenously within 3 hours before the operation, for 7 days. For acute liver failure, cefotaxime (100 mg/kg per day) plus acyclovir (30 mg/kg per day) was administered at the time of diagnosis and then switched to the regular regimen after LT. | Induction: oral tacrolimus (0.075 mg/kg) intravenous basiliximab (12 mg/m2) methylprednisolone (20 mg/kg) |
| For maintenance immunosuppression, oral tacrolimus was tapered to a dosage to maintain trough levels of <5 ng/mL. The oral prednisolone was also tapered and then stopped around 3–6 mo postoperatively. | |||
| Béranger et al., [15] 2020 | France | Cefoxitin was used as standard antibiotic prophylaxis in patients operated from 2009 to 2011. Piperacillin-tazobactam was used thereafter. Imipenem was used for patients with ESBL-PE stool carriage. Two days of prophylaxis was started at the beginning of the surgery, with a repeat intraoperative dose in cases of bleeding over 1 blood volume or surgery lasting over 6 hours. Prophylaxis was prolonged for 5 days after LT in cases of bowel wounds or if a prosthetic mesh was used for abdominal wall closure. Patients with pre-operative methicillin-resistant S. aureus nasal carriage were treated with topical mupirocin before LT and by vancomycin during surgery. Penicillin prophylaxis was continued for patients with polysplenia. | Induction: basiliximab and tacrolimus |
| If needed, mycophenolate mofetil was added as a renalsparing agent or to increase immunosuppression. | |||
| Balcı Sezer et al., [16] 2020 | Turkey | Ampicillin (200 mg/kg/day) and cefotaxime (150 mg/kg/day) for 72 hours | The standard immunosuppressive regimen after LT con sisted of tacrolimus and mycophenolate mofetil. |
| Methylprednisolone was started intraoperatively (10 mg/kg/dose) and continued with tapering for the first 3 months after LT. | |||
| Phichaphop et al., [17] 2020 | Thailand | Ampicillin/sulbactam (200 mg/kg/day of ampicillin) and ceftriaxone (50 mg/kg/day) | Induction: tacrolimus, corticosteroid, and mycophenolate mofetil |
| Dohna Schwake et al., [19] 2020 | France | Perioperative and postoperative antibiotic prophylaxis ticarcilline/clavulanic acid 75 mg/kg tid for 48 hours | Induction: tacrolimus |
| If diuresis was present, 2 doses of | |||
| basiliximab were administered on day 0 and 4 after transplantation, respectively. | |||
| Corticosteroids were not administered on a routine basis; In selected patients with impaired renal function, mycophenolate mofetil (20 mg/kg/day) was added to tacrolimus. | |||
| Duncan et al., [20] 2019 | South Africa | Perioperative antibacterial prophylaxis theater with piperacillin/tazobactam (100 mg/kg/dose, 8 hourly) and continued for 24 hour postoperatively. For children transplanted for acute liver failure, the antibacterial prophylaxis was continued for 7 days, with additional antifungal prophylaxis in the form of micafungin (150 mg/kg/dose) for the same duration. | Corticosteroids and tacrolimus and Intravenous methylprednisolone was administered intraoperatively at transplant, followed by oral prednisone from day 1 which was tapered and stopped within 6 months posttransplant. |
| Pouladfar et al., [22] 2019 | Iran | Ampicillin-sulbactam (150 mg/kg/day) and ceftizoxime (150 mg/kg/day) for 72 hours in all patients, except in those with bilo enteric anastomosis (continued for 5 days). | Induction: tacrolimus, mycophenolate mofetil, and steroids. |
| Furuichi et al., [23] 2018 | Japan | Ampicillin (120 mg/kg/day, q6hrs) and cefotaxime (120 mg/kg/day, q6hrs) were administered intravenously within 1 hour before LT and continued for 48 hours after surgery. Perioperative prophylactic regimen was also modified according to the patient’s history of infection or colonization. | Tacrolimus and steroids |
| Recipients older than or equal to 1 year of age with ABOincompatible donors received Rituximab 2 weeks before LT and mycophenolate mofetil after LT. | |||
| Shepherd et al., [5] 2013 | USA | The regular perioperative prophylaxis consisted of ampicillin (120 mg/kg/day, q6hrs) and cefotaxime (120 mg/kg/day, q6hrs) administered intravenously within 1 hour before the LT and continued for 48 hours. | Not mentioned. |
| Nafady-Hego et al., [24] 2011 | Japan | Flomoxef was given to patients 1 hour before the operation and continued for 72 hours thereafter. Trimethoprim and sulfamethoxazole were administered once daily as a prophylaxis against Pneumocystis. Miconazole oral gel was administered for 7 days after transplantation as antifungal prophylaxis. | Tacrolimus and corticosteroid |
| In the case of ABO incompatibility, prophylactic steroid pulse was administered every week for the first month. Prostaglandin E1 was infused for 7–14 days after LT. | |||
| Cyclophosphamide therapy was initiated 1 week before transplant and given daily for 1 month after LT, and was then converted to azathioprine |
Table 4.
Risk factors for BSI among pediatric LT patients
| Study | Type of pathogen in BSI | Risk factors | |
|---|---|---|---|
| Kim et al., [13] 2022 | Bacterial and Fungal | Univariate: | |
| Age, z score of height, z score of weight, the presence of growth failure, etiology biliary atresia, liver support system, total volume of RBC transfusion, post-LT hospital stay, portal vein complication, reoperation | |||
| Multivariate: | |||
| Age of ≤1.3 years, combined growth failure, experience with a liver support system, longer hospital stay of > 44 days | |||
| Dohna Schwake et al., [19] 2020 | Bacterial | Multivariate: | |
| - RF for severe sepsis or septic shock: colonization with MDR bacteria, tacrolimus level >20 ng/mL, cold ischemia time, chronic kidney injury | |||
| - RF for CLABSI: cold ischemia time, age, male sex, central catheter days | |||
| Duncan et al., [20] 2019 | Bacterial and Fungal | Univariate: | |
| BA as a cause of liver failure, the development of postoperative biliary complications | |||
| Shoji et al., [21] 2019 | Bacterial and Fungal | Univariate: | |
| - ABO incompatibility, PELD/MELD score, blood volume loss during LT, positivity of CMV antigenemia after | |||
| LT | |||
| - RF unique to patients ≤24 months: age, body weight, number of operations before LT | |||
| - RF unique to patients >24 months: graft-recipient body weight ratio, operative time | |||
| Multivariate: | |||
| - Body weight, blood volume loss during LT, positivity of CMV antigenemia after LT, PELD/MELD score | |||
| - RF unique to patients ≤24 months: PELD/MELD score | |||
| Furuichi et al., [23] 2018 | Bacterial | Univariate: | |
| Blood loss >60 mL/kg, prolonged operative time >12 hours, history of early-onset BSI, prolonged hospital stay more than 6 months, biliary stenosis, histological rejection during 6 months after LT | |||
| Multivariate: | |||
| Prolonged operative time >12 hours, biliary stenosis | |||
References
1. National OPTN data (all donors) [Internet]. OPTN Metrics; [cited 2023 Oct 10]. Available from: https://insights.unos.org/OPTN-metrics/.
2. Cananzi M, Gaio P, Boscardin C, Pescarin M, Bosa L.Indications to liver transplantation in children.In: Burra P, editor. Textbook of liver transplantation. Cham (Switzerland): Springer; 2022:495-507.
3. National Healthcare Safety Network. Bloodstream infection event (central line-associated bloodstream infection and noncentral line associated bloodstream infection) [Internet]. Atlanta (GA): Centers for Disease Control and Prevention; 2023 [cited 2023 Oct 27]. Available from:https://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf.
4. Bouchut JC, Stamm D, Boillot O, Lepape A, Floret D. Postoperative infectious complications in paediatric liver transplantation: a study of 48 transplants. Paediatr Anaesth 2001;11:93-8.


5. Shepherd RW, Turmelle Y, Nadler M, Lowell JA, Narkewicz MR, McDiarmid SV, et al. Risk factors for rejection and infection in pediatric liver transplantation. Am J Transplant 2008;8:396-403.



6. Araz C, Pirat A, Torgay A, Zeyneloglu P, Arslan G. Early postoperative complications of pediatric liver transplantation: experience at one center. Transplant Proc 2004;36:214-7.


7. Green M, Michaels MG. Infections in pediatric solid organ transplant recipients. J Pediatric Infect Dis Soc 2012;1:144-51.


8. Kritikos A, Manuel O. Bloodstream infections after solid-organ transplantation. Virulence 2016;7:329-40.



9. Pappas PG, Alexander BD, Andes DR, Hadley S, Kauffman CA, Freifeld A, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis 2010;50:1101-11.

10. Husain S, Tollemar J, Dominguez EA, Baumgarten K, Humar A, Paterson DL, et al. Changes in the spectrum and risk factors for invasive candidiasis in liver transplant recipients: prospective, multicenter, case-controlled study. Transplantation 2003;75:2023-9.


11. Nagao M, Fujimoto Y, Yamamoto M, Matsumura Y, Kaido T, Takakura S, et al. Epidemiology of invasive fungal infections after liver transplantation and the risk factors of late-onset invasive aspergillosis. J Infect Chemother 2016;22:84-9.


12. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71.



13. Kim YE, Choi HJ, Lee HJ, Oh HJ, Ahn MK, Oh SH, et al. Assessment of pathogens and risk factors associated with bloodstream infection in the year after pediatric liver transplantation. World J Gastroenterol 2022;28:1159-71.



14. Sintusek P, Poovorawan Y. Immunization status and hospitalization for vaccine-preventable and non-vaccine-preventable infections in liver-transplanted children. World J Hepatol 2021;13:120-31.



15. Béranger A, Capito C, Lacaille F, Ferroni A, Bouazza N, Girard M, et al. Early bacterial infections after pediatric liver transplantation in the era of multidrug-resistant bacteria: nine-year single-center retrospective experience. Pediatr Infect Dis J 2020;39:e169-75.

16. Balcı Sezer O, Barı Z, Ecevit Z, Özçay F, Haberal M. Prevalence of Infections in Infants Within the First 6 Months of Liver Transplant. Exp Clin Transplant 2020;18(Suppl 1): 93-5.

17. Phichaphop C, Apiwattanakul N, Techasaensiri C, Lertudomphonwanit C, Treepongkaruna S, Thirapattaraphan C, et al. High prevalence of multidrug-resistant gram-negative bacterial infection following pediatric liver transplantation. Medicine (Baltimore) 2020;99:e23169.



18. Alcamo AM, Alessi LJ, Vehovic SN, Bansal N, Bond GJ, Carcillo JA, et al. Severe sepsis in pediatric liver transplant patients: the emergence of multidrug-resistant organisms. Pediatr Crit Care Med 2019;20:e326-32.



19. Dohna Schwake C, Guiddir T, Cuzon G, Benissa MR, Dubois C, Miatello J, et al. Bacterial infections in children after liver transplantation: a single-center surveillance study of 345 consecutive transplantations. TransplInfectDis 2020;22:e13208.
20. Duncan M, DeVoll-Zabrocki A, Etheredge HR, Maher HA, Bouter C, Gaylard P, et al. Blood stream infections in children in the first year after liver transplantation at Wits Donald Gordon Medical Centre, South Africa. Pediatr Transplant 2020;24:e13660.



21. Shoji K, Funaki T, Kasahara M, Sakamoto S, Fukuda A, Vaida F, et al. Risk factors for bloodstream infection after living-donor liver transplantation in children. Pediatr Infect Dis J 2015;34:1063-8.



22. Pouladfar G, Jafarpour Z, Malek Hosseini SA, Firoozifar M, Rasekh R, Khosravifard L. Bacterial infections in pediatric patients during early post liver transplant period: A prospective study in Iran. Transpl Infect Dis 2019;21:e13001.



23. Furuichi M, Fukuda A, Sakamoto S, Kasahara M, Miyairi I. Characteristics and risk factors of late-onset bloodstream infection beyond 6 months after liver transplantation in children. Pediatr Infect Dis J 2018;37:263-8.


24. Nafady-Hego H, Elgendy H, Moghazy WE, Fukuda K, Uemoto S. Pattern of bacterial and fungal infections in the first 3 months after pediatric living donor liver transplantation: an 11-year single-center experience. Liver Transpl 2011;17:976-84.



25. Kim JE, Oh SH, Kim KM, Choi BH, Kim DY, Cho HR, et al. Infections after living donor liver transplantation in children. J Korean Med Sci 2010;25:527-31.




26. Graziadei I, Zoller H, Fickert P, Schneeberger S, Finkenstedt A, Peck-Radosavljevic M, et al. Indications for liver transplantation in adults: Recommendations of the Austrian Society for Gastroenterology and Hepatology (ÖGGH) in cooperation with the Austrian Society for Transplantation, Transfusion and Genetics (ATX). Wien Klin Wochenschr 2016;128:679-90.




27. Kolesnichenko SI, Lavrinenko AV, Akhmaltdinova LL. Bloodstream infection etiology among children and adults. Int J Microbiol 2021;2021:6657134.









 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


