Article Contents
| Clin Exp Pediatr > Volume 67(6); 2024 |
|
Abstract
Action-plan is a written set of instructions that helps patient manage their symptoms and respond to worsening of their condition. The action-plan usually includes information on how to recognize, treat, and prevent worsening of symptoms. The plan also helps patient understand when to use their medications, how much to use, and how often to use them as-needed. An action-plan should be developed through a discussion between the patient and the physician, reflecting the patient's severity, preferences, and values and should be regularly updated to reflect changes in the person's condition. In asthma, action-plans and as-needed therapy are already well utilized. Unlike asthma, the importance of an action-plan has been overlooked in allergic rhinitis (AR), but its importance has recently been recognized. AR is a chronic condition that affects people differently, and can cause a range of symptoms, including nasal congestion, runny nose, sneezing, itching, and watery eyes. Therefore, an action-plan and as-needed therapy can help patients manage these symptoms more effectively, reducing the impact on their daily activities and quality of life. Furthermore, it can be tailored to meet the personal needs of each patient, based on the severity of their symptoms, their triggers, and their overall health. Because action-plan can help patients adhere to their treatment regimen by providing clear instructions on when and how to take medication, it can help patients stay on track with their treatment, reducing the likelihood of missed doses and treatment failures. Overall, an action-plan and as-needed therapy are important components of a comprehensive treatment plan for patients with AR. They can help to improve symptom control, prevent complications, and promote adherence to treatment, leading to better outcomes and a higher quality of life.
Allergic rhinitis (AR) is a major chronic respiratory disease due to its prevalence, impact on quality of life (QoL), impact on work or school performance and productivity, economic burden, and links with asthma. The prevalence and the socioeconomic burden associated with the medical cost and QoL of AR have progressively increased worldwide.
Most of evidence-based guidelines for AR propose identification and avoidance of triggers, long-term continuous or step-wised therapy for patients with persistent symptoms or intermittent therapy for whom with intermittent symptoms, and allergen immunotherapy for patients with persistent and severe AR despite appropriate management [1-6]. However, these guidelines are mostly based on randomized controlled trials (RCTs), may not reflect real world [7,8]. Many patients do not achieve symptom control with guideline- based, continuous therapy, or have symptoms that are too mild or often variable depending on their trigger and comorbidity. Currently available treatments for AR include antihistamines, intranasal corticosteroids (INCS), leukotriene receptor antagonist(LTRA), alpha-sympathomimetics (decongestants) and immunotherapy. While these therapies can be effective for many people, they may not work for everyone, and they can also cause side effects such as drowsiness, dry mouth, and nasal irritation [8,9]. Therefore, practical guidelines for the appropriate management of AR need to be developed based on scientific evidence while considering the real-world environment, values, and preferences of patients and physicians.
In the Allergic Rhinitis and its Impact on Asthma (ARIA) classification, AR is divided into intermittent and persistent according to the duration of symptoms, and mild and moderate/severe according to the impact on daily life [1]. The ARIA guideline emphasizes the importance of properly diagnosing and treating AR, which is a common condition that affects up to 30% of the population. It also highlights the significant impact that AR can have on asthma, as up to 80% of people with asthma also have AR. The guideline recommends a stepwise approach to treatment, starting with the use of allergen avoidance strategies and pharmacotherapy. For people with moderate to severe AR, immunotherapy is recommended. The position paper of the European Academy of Allergy and Clinical Immunology also recommend a stepwise approach and add-on therapy depending on the severity of symptoms and responsibility for the given treatment in pediatric population [10].
The ARIA guideline also emphasizes the importance of patient education and self-management, as well as the need for multidisciplinary care that includes allergists, pulmonologists, and primary care providers. Physicians think they explain enough to their patients and trust them to follow their prescriptions, but the real world is different. The MASK-air data suggests that the guidelines may not work in the real world [11,12]. Bousquet et al. [13] highlights that many patients are dissatisfied with their treatment, with symptoms remaining poorly controlled. Moreover, adherence to treatment is usually poor in AR. Patients treat themselves according to the symptoms, and comedication use is driven by symptom severity. Many patients are ondemand users, but guidelines have not yet approached this issue.
In Korea, AR for pollen or pets is dramatically increasing [14,15]. Pollen induces a typical pattern of seasonal worsening, and pets cause both episodic intermittent and persistent AR. AR is a complex disease with many different underlying causes, and there is currently no one-size-fits-all treatment approach. There is a need for more personalized treatment approaches that take into account a patient's individual symptoms, medical history, and underlying causes.
In this paper, I would like to discuss the unmet need of patients with AR in the real-world and need for as-needed therapy according to the action-plan of AR. As-needed therapy include use of symptom-reliever on worsening of symptoms and use of symptom-preventer for anticipated worsening.
MASK-air (Mobile Airways Sentinel NetworK for airway diseases) is an mHealth application, provides a real world evidence from patients-centered practice [16,17]. Recently it shows an unmet needs of patients and gaps between real-world practice and guidelines [18]. Briefly, MASK air data show that most rhinitis patients (1) are not adherent and do not follow guidelines-based therapy, (2) often prefers an as needed therapy, and (3) more likely to self-determine length of treatment based on their symptoms. In detail, MASK-air shows (1) that during 222,024 days by 13,122 users, 63,887 days (28.8%) were under monotherapy and 38,315 (17.3%) under comedication, while 119,823 days (54.0%) were under no therapy [11], (2) that a large number of patients in Europe reported an annual average of 2.7 drugs with 80% reporting 2 or more drugs, particularly during spring-pollen season; the most-common as-needed therapy was oral antihistamines (OAH) [11,19], (3) that 69.05% of users were nonadherent to medications, while only 11.28% were adherent and 4.23% partly adherent [12], and (4) that switching of medications was common, particular in uncontrolled weeks [18,20]. These findings suggest that patients treat themselves according to their symptoms and comedication used is driven by symptoms severity and self-decision. Recent expert opinion suggests that there is urgent need for change management and proposed need for as-needed treatment reflecting patient’s need [13]. Unfortunately, the MASK-air did not showed a results from pediatric population.
many patients with AR report that their current medications take too long to provide relief from their symptoms. Patients want medications that work quickly to relieve symptoms and allow them to resume their daily activities.
Patients also want medications that provide longer-lasting relief from their symptoms, so they do not have to take medication as frequently or worry about symptoms returning quickly.
Many medications for AR can cause side effects, such as drowsiness or dry mouth, which can interfere with daily activities or work. Patients want medications that are well-tolerated and have minimal side effects.
Patients may have difficulty remembering to take medication regularly or may find it inconvenient to take medication multiple times a day. Patients want medications with more convenient dosing regimens, such as once-daily dosing.
Parents want age-appropriate medication formulations and dosing to ensure that their child receives the correct amount of medication for their age and size.
Most evidence-based, clinical practice guideline are based on RCTs, which are typically conducted in select populations. RCTs are the best way of assessing size of a treatment effect and are necessary not only for drug registration but also to establish general and objective evidence levels and recommendations. However, there are limitations to generalizing the results of RCTs to the real world [7]. These guidelines all suggest long-term, continuous treatment. Nonetheless, many patients are dissatisfied with their treatment, with symptoms remaining poorly controlled [8]. While most doctors believe that if they follow the guidelines, their patients will be well controlled, most parents reported that they or their children were unhappy with the incomplete relief of symptoms provided by their current nasal allergy medication [21], as well as adult patients [13]. Recent mHealth MASK-air highlights that many patients are dissatisfied and nonadherent for treatment by evidence-based guideline and symptoms remaining poorly controlled, patients are likely to treat themselves according to symptoms and comedication use is frequent in proportion to severity, and many patients prefer as-needed therapy, but guidelines do not provide guidance on as-needed therapy [11,19,24]. Guidelines have not yet approached this issue, although guidelines strive to reflect real world evidence to compensate for these limitations.
The ARIA guidelines 2010 revision and International Consensus Statement on Allergy and Rhinology: Allergic Rhinitis (ICAR-Allergic rhinitis 2023) made a strong recommendation for INCS or newer-generation antihistamines for the first-line treatment [1,4]. INCS is a most effective anti-inflammatory treatment for AR, but it has a disadvantage of slow onset of action. While, newer-generation antihistamines have an advantage of rapid onset of action, but they do not have anti-inflammatory effect. To overcome these disadvantages and maximize the advantage, combination drugs such as INCS/INAH are available, but they also have various disadvantages in terms of patients’ preference.
In the aspects of onset of action, topical decongestants are the fastest acting, but have serious side effects. First-generation antihistamines have anticholinergic side effects and can cross the blood–brain barrier, resulting in central nervous system effects such as sedation and drowsiness. Newer-generation antihistamines block peripheral H1 receptors without crossing the blood–brain barrier which prevents central nervous system side effects. cetrizine and levocetrizine have a faster onset of action (0.7 hour) than loratadine, desloratadine, and fexofenadine (onset of action >1 hour) [4]. As described above, fast and long-lasting therapeutic response the most important need for patients.
Allergic Rhinitis founded by American Academy of Otolaryngology-Head and Neck Surgery added a concept of episodic AR. This concept defined AR as a disease caused by an immunoglobulin E-mediated inflammatory response that can occur if an individual is in contact with an exposure that is not normally a part of the individual’s environment. (i.e., a cat at a friend’s house) [2]. This guideline also recommended a INCS as a first-line treatment in both seasonal and perennial AR, but recommend an INAH as first-line treatment in episodic AR. ICAR-Allergic Rhinitis 2023 proposed that the decision on which medications for AR to prescribe should be personalized to the patient and the dosing, drug interactions, side effects, the onset of action, and cost should be considered.
Recently, the Korean Academy of Asthma, Allergy and Clinical Immunology revised clinical guidelines of AR to address key clinical questions of the management of AR [3,5]. Part 1 of the revised guideline covers the pharmacological management and part 2 covers nonpharmacological management of patents with AR in Korea. These guidelines provide recommendation focusing on the balance between benefit and harms, and patients’ preference, based on the evidence fromRCTs.To some extent,these guidelines reflect the needs of the real world, but they do not fully reflect the real-world’s needs because they are based on evidence from RCTs. Table 1 summarizes the recommendation of KAAACI(Korean Academy of Asthma, Allergy and Clinical Immunology) AR guidelines. These guidelines recommend INCS and intranasal antihistamine (INAH) combination therapy or adding OAHs on the INCS for the patients who were under controlled with INCS monotherapy considering the patient’s values and preferences as well as the benefits and harms of treatment [25]. In addition, it recommends preventing therapy 2 weeks before pollen dispersal in patients with pollen-induced AR.
As-needed therapy may have 2 concepts of therapy; symptom-reliever and symptom-preventer. The first one is using medication to relieve symptoms as they occur. For example, a patient may use an OAH, INAH, or INCS when they experience sneezing, runny nose, and itchy eyes. As-needed therapy with symptom relieving can be effective for patients with mild or intermittent symptoms, and combination with continuous treatment can increase adherence and satisfaction of patients. Second one may be defined as as-needed therapy with symptom preventing, particular in episodic intermittent AR.
Guideline-based management of AR recommend a therapy involves using continuous medication on a regular basis to control symptoms and inflammation (e.g., continuous using of INCS in perennial AR). This type of therapy is recommended for patients with moderate to severe symptoms or those who experience symptoms year-round. As-needed therapy involves a real prophylactic using to prevent worsening of symptoms in predictable situations (e.g., using medication before exposure to pets or pollen) or relieving symptoms. On or before day exposing pets, use of oral antihistamine or INAH would be better than use of INCS. On the other hand, starting INCS and combination with other medications before 2 weeks of pollen dispersal in patients with pollen-induced AR [5]. Sometimes, patients prefer symptoms-relieving therapy than symptoms-preventing one, particular in mild symptoms. Ultimately, the choice between continuous therapy and as-needed therapy will depend on the personal symptoms and needs.
Hoang et al. [26] reported a systematic review and meta-analysis including 8 studies (882 participants) for RCTs studying the effects of as-needed INCS compared to regular INCS. It showed that as-needed INCS showed inferiority than regular INCS in total nasal symptom score (TNSS), disease-specific QoL, nasal peak inspiratory flow, sneezing, and nasal congestion scores, but showed no difference in ocular symptoms, symptom-free days, nasal itching, and rhinorrhea scores. Furthermore, there was no difference in TNSS in the pediatric population (one study including 46 participants).
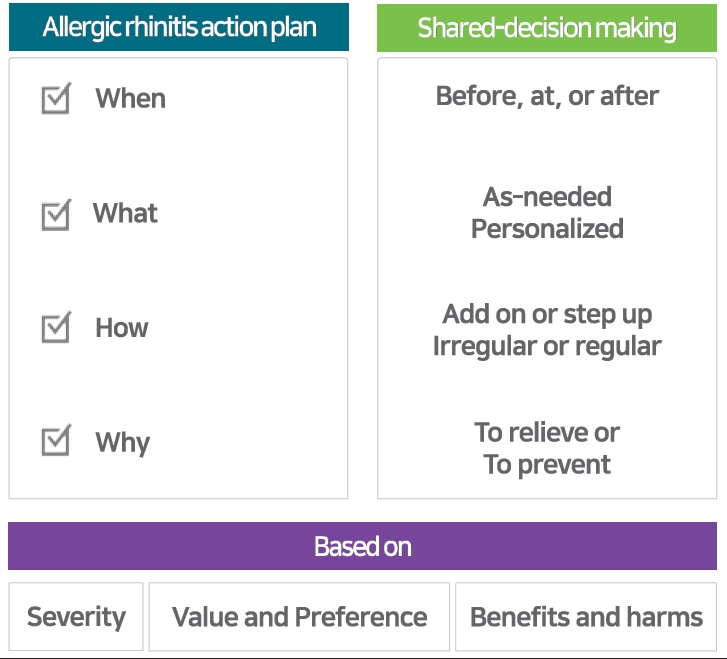
An AR action-plan is a written set of instruction that helps patients with AR manage their symptoms and respond to worsening of their symptoms. Based on the patient’s severity and variable of symptoms, values and preferences as well as the benefits and harms of treatment. Action-plan has to includes the following elements; when, what, how, and why (Table 2).
The action-plan includes a concept of “when” according to the patient’s type of AR. The “when” of perennial AR means year-round, 2 weeks before and during pollen season would be “when” of seasonal AR. In episodic intermittent AR, the “when” would be immediately before and after the exposure. The action-plan also includes how avoid or reduce a trigger and how manage AR. Medication planning depends on the severity of the symptoms and the type of AR (e.g., perennial, seasonal, or episodic). Mild symptoms may be managed with lifestyle modifications and intermittent therapy using OAHs, while more severe symptoms may require continuous or combination therapy. The specific allergens that trigger the patient's symptoms will also be included in action-planning. Allergen testing may be necessary to identify the triggers, and avoidance strategies should be recommended. Patients' lifestyle or environmental factors, such as their work schedule or travel plans, may also be considered when developing a personalized action plan. For example, patients who travel to a country during pollen season on the other side of the world, or who plan to visit to pet-owners’ house.
The action-plan for perennial (e.g., house dust mite, HDM), seasonal (e.g., pollen), and episodic intermittent (e.g., dog or cat) may have some similarities, but may also have some differences based on the specific triggers and symptoms that are experienced. The action-plan for HDM may focus on more measures to reduce exposure to HDM in indoor during year-round, however those for pollen may focus on avoidance measure in outdoor during a specific pollen season.Although level of evidence is not strong,allergy-proof bedding, removing carpet, use of high efficiency particulate air filter, and keeping pets out of bedroom are common plan for AR patients [3]. However, frequent ventilation, reducing humidity below 50% are recommended in a patient triggered by HDM.In pollen AR, avoidance outdoor activity, less ventilation, changing clothes and bathing after outdoor activity, and monitoring pollen counts using an application during pollen season differ from whom with HDM allergy.
For moderate to severe, perennial or seasonal AR, INCS may firstly be recommended. However, intermittent combination therapy is necessary for patients who are not adequately controlled with INCS alone. On the contrary, moderate to severe episodic AR needs a rapid-acting as-need therapy, not an INCS. For example, taking symptoms preventer (e.g., rapid onset oral antihistamines) before or symptoms relievers (e.g., INAH, INAH/INCS, OAH, or decongestants) after visiting a pet owner’s house. The action-plan should include the choice and combination of medication depending on mechanisms and effects of medications, time and intensity of exposure, and personal sensitivity. Medications for AR have a various range of onset of action; topical decongestants (within 10 minutes), INAH (15 minutes), OAH (0.7–3 hours), INCS (~several days), LTRA (~several days). INCS and LTRA have an antiinflammatory effect, but others do not have. Sneezing and itching should include antihistamines. The INCS and INAH combination therapy can provide faster and more effective symptoms relief than using either medication alone. However, it should be considered about poor taste in the aspect of patient’s preference [25]. Some patients may have preferences or concerns about certain medications, such as concerns about side effects or a preference for natural remedies. It is important for physicians to take these preferences into account when developing a action plan for patients with AR. Providers can discuss the benefits and harms of different medication options with their patients, as well as provide education on non-pharmacologic strategies for managing symptoms. Ultimately, the best treatment plan is one that meets the personal needs and preferences.
Overall, personalized action plans for AR should be tailored to the personal needs and preferences, and may include a combination of lifestyle modifications and medications. Regular follow-up with a physician can help ensure that the action plan is working effectively and can be adjusted as-needed.
AR can be particularly challenging to manage in children, who may have difficulty communicating their symptoms and may be more prone to side effects from medications. Unfortunately, due to the lack of pediatric studies, this review is based primarily on studies in adults, not children. There is growing need for research reflecting needs, preference, and values of pediatric patients.
Table 1.
Summary of recommendations for management of allergic rhinitis
Table 2.
Key elements of allergic rhinitis action-plan
References
1. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy 2008;63 Suppl 86:8–160.

2. Seidman MD, Gurgel RK, Lin SY, Schwartz SR, Baroody FM, Bonner JR, et al. Clinical practice guideline: Allergic rhinitis. Otolaryngol Head Neck Surg 2015;152(1 Suppl): S1–43.
3. Park DY, Lee YJ, Kim DK, Kim SW, Yang HJ, Kim DH, et al. KAAACI Allergic Rhinitis Guidelines: Part 2. Update in nonpharmacological management. Allergy Asthma Immunol Res 2023;15:145–59.




4. Wise SK, Damask C, Roland LT, Ebert C, Levy JM, Lin S, et al. International consensus statement on allergy and rhinology: Allergic rhinitis - 2023. Int Forum Allergy Rhinol 2023;13:293–859.

5. Yang SI, Lee IH, Kim M, Ryu G, Kang SY, Kim MA, et al. KAAACI Allergic Rhinitis Guidelines: Part 1. Update in pharmacotherapy. Allergy Asthma Immunol Res 2023;15:19–31.




6. Okubo K, Kurono Y, Ichimura K, Enomoto T, Okamoto Y, Kawauchi H, et al. Japanese guidelines for allergic rhinitis 2020. Allergol Int 2020;69:331–45.


7. Travers J, Marsh S, Williams M, Weatherall M, Caldwell B, Shirtcliffe P, et al. External validity of randomised controlled trials in asthma: to whom do the results of the trials apply? Thorax 2007;62:219–23.



8. Price D, Smith P, Hellings P, Papadopoulos N, Fokkens W, Muraro A, et al. Current controversies and challenges in allergic rhinitis management. Expert Rev Clin Immunol 2015;11:1205–17.


9. Ferrando M, Bagnasco D, Varricchi G, Bernardi S, Bragantini A, Passalacqua G, et al. Personalized Medicine in Allergy. Allergy Asthma Immunol Res 2017;9:15–24.




10. Roberts G, Xatzipsalti M, Borrego LM, Custovic A, Halken S, Hellings PW, et al. Paediatric rhinitis: position paper of the European Academy of Allergy and Clinical Immunology. Allergy 2013;68:1102–16.


11. Sousa-Pinto B, Sá-Sousa A, Vieira RJ, Amaral R, Klimek L, Czarlewski W, et al. Behavioural patterns in allergic rhinitis medication in Europe: A study using MASK-air(®) real-world data. Allergy 2022;77:2699–711.

12. Menditto E, Costa E, Midão L, Bosnic-Anticevich S, Novellino E, Bialek S, et al. Adherence to treatment in allergic rhinitis using mobile technology. The MASK Study. Clin Exp Allergy 2019;49:442–60.

13. Bousquet J, Toumi M, Sousa-Pinto B, Anto JM, Bedbrook A, Czarlewski W, et al. The Allergic Rhinitis and Its Impact on Asthma (ARIA) approach of value-added medicines: as-needed treatment in allergic rhinitis. J Allergy Clin Immunol Pract 2022;10:2878–88.

14. Oh JW. Pollen allergy in a changing planetary environment. Allergy Asthma Immunol Res 2022;14:168–81.




15. Park YB, Mo EK, Lee JY, Kim JH, Kim CH, Hyun IG, et al. Association between pet ownership and the sensitization to pet allergens in adults with various allergic diseases. Allergy Asthma Immunol Res 2013;5:295–300.



16. Bousquet J, Anto JM, Bachert C, Haahtela T, Zuberbier T, Czarlewski W, et al. ARIA digital anamorphosis: digital transformation of health and care in airway diseases from research to practice. Allergy 2021;76:168–90.

17. Bousquet J, Bedbrook A, Czarlewski W, Onorato GL, Arnavielhe S, Laune D, et al. Guidance to 2018 good practice: ARIA digitally-enabled, integrated, person-centred care for rhinitis and asthma. Clin Transl Allergy 2019;9:16.


18. Bousquet J, Anto JM, Sousa-Pinto B, Czarlewski W, Bedbrook A, Haahtela T, et al. Digitally-enabled, patient-centred care in rhinitis and asthma multimorbidity: the ARIA-MASK-air(®) approach. Clin Transl Allergy 2023;13:e12215.


19. Sousa-Pinto B, Schünemann HJ, Sá-Sousa A, Vieira RJ, Amaral R, Anto JM, et al. Comparison of rhinitis treatments using MASK-air® data and considering the minimal important difference. Allergy 2022;77:3002–14.

20. Sousa-Pinto B, Schünemann HJ, Sá-Sousa A, Vieira RJ, Amaral R, Anto JM, et al. Consistent trajectories of rhinitis control and treatment in 16,177weeks: the MASK-air® longitudinal study. Allergy 2023;78:968–83.

21. Meltzer EO, Blaiss MS, Derebery MJ, Mahr TA, Gordon BR, Sheth KK, et al. Burden of allergic rhinitis: results from the Pediatric Allergies in America survey. J Allergy Clin Immunol 2009;124(3 Suppl): S43–70.


22. Bousquet J, Hellings PW, Agache I, Amat F, Annesi-Maesano I, Ansotegui IJ, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) Phase 4 (2018): change management in allergic rhinitis and asthma multimorbidity using mobile technology. J Allergy Clin Immunol 2019;143:864–79.

23. Yang HJ, Kim YH, Lee B, Kong DY, Kim DK, Kim MA, et al. Unmet primary physicians' needs for allergic rhinitis care in Korea. Allergy Asthma Immunol Res 2017;9:265–71.




24. Sousa-Pinto B, Anto A, Berger M, Dramburg S, Pfaar O, Klimek L, et al. Real-world data using mHealth apps in rhinitis, rhinosinusitis and their multimorbidities. Clin Transl Allergy 2022;12:e12208.







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


