Article Contents
| Clin Exp Pediatr > Volume 68(4); 2025 |
|
Abstract
Cerebral organoids derived from human induced pluripotent stem cells offer a groundbreaking foundation for the analysis of pediatric neurological diseases. Unlike organoids from other somatic systems, cerebral organoids present unique challenges, such as the high sensitivity of neuronal cells to environmental conditions and the complexity of replicating brain-specific architectures. Cerebral organoids replicate the human brain development and pathology, enabling research on conditions such as microcephaly, Rett syndrome, autism spectrum disorders, and brain tumors. This review explores the utility of cerebral organoids for modeling diseases and testing therapeutic interventions. Despite current limitations such as variability and lack of vascularization, recent technological advancements have improved the reliability and application of such interventions. Cerebral organoids provide valuable insight into the mechanisms underlying complex neural disorders and hold promise as novel treatment strategies for pediatric neurological diseases.
Graphical abstract
Achieving the proper diagnosis and treatment of pediatric diseases requires in-depth knowledge of physiological and pathological processes. Animal and cell culture models played crucial roles in this study. Mouse models are widely used for convenience and their chemical, molecular, cellular, and anatomical similarities to humans [1]. Many human diseases affect differentiated cell types, including neurons and somatic cells, which are challenging to sample, culture, and sustain. Even when primary cell lines are generated from tissues, obtaining significant results is often hindered by issues related to replicability, dedifferentiation, and variability caused by the culture conditions. In 2006, Takahashi and Yamanaka [2] first demonstrated that murine skin cells can be transformed into embryonic stem (ES)-like cells through the introduction and expression of specific genes using a viral transfection vector. This breakthrough has been validated by several research groups, who confirmed the reprogramming of skin cells into ES-like cells or induced pluripotent stem cells (iPSCs), requiring only 4 genes (OCT3/4, SOX2, KLF4, and C-MYC) [2,3]. In fact, iPSCs have become a leading basic research tool in cancer, neurological diseases, human development, and various other fields. Neurological disorders arise from defects in various nervous system cell types such as neurons, astrocytes, and glial cells, all of which can be generated using current iPSC differentiation protocols. Progress in tissue engineering may enable the creation of organoid models that replicate the intricate cell networks of the brain, spinal cord, and peripheral nervous system.
Amyotrophic lateral sclerosis (ALS) [4] is characterized by the progressive degeneration of the upper and lower motor neurons in the brain and spinal cord. Motor neurons derived from iPSCs from patients with ALS successfully mimic the ALS phenotype. Spinal muscular atrophy (SMA) [5], an autosomal recessive disorder affecting the voluntary skeletal muscles, is among the first genetic disorders successfully modeled using iPSCs [6,7], replicating the effects of SMA on neuromuscular junctions. Psychiatric disorders such as schizophrenia and bipolar disorder have also been studied using iPSCs. Schizophrenia is a complex debilitating psychiatric disorder that typically manifests as psychosis during early adulthood. The causes of schizophrenia are multifaceted and involve genetic predisposition and environmental factors. Schizophrenia affects high-level neurobehavioral functions, and its complex genetic influences have complicated the development of valid animal models. Neurons derived from iPSCs of patients with schizophrenia have provided insight into its pathogenesis by identifying genetic risk factors and altered signaling pathways [8,9].
Notably, specific phenotypic markers such as reduced neuronal connectivity and glutamate expression were reversed in iPSC models of schizophrenia following treatment with antipsychotic drugs [10]. Additionally, an iPSC model of bipolar disorder demonstrated altered neurogenesis and neuroplasticity, a phenotype that was restored by pharmacological intervention [11]. Findings from iPSC models of bipolar disorder may be instrumental in the development of new and optimization of existing treatment options. Rett syndrome, a severe neurodevelopmental condition resulting from mutations in the MECP2 gene, has also been studied using iPSCs. Neurons derived from iPSCs of patients with Rett syndrome showed phenotypic differences, including smaller size, fewer synapses, and abnormal signaling, compared to controls. Pharmacological treatments correct synaptic abnormalities and highlight a potential developmental window for therapeutic interventions [12].
Building on advancements in iPSC technology, further progress has been made in replicating brain architecture. Although organoids represent a more recent breakthrough in this field, spheroids and rosettes have been crucial, particularly in cancer and neuroscience research. The key distinctions between organoids and spheroids lie in the more advanced level of self-organization observed in organoids and their reliance on matrix-like substances, such as Matrigel or collagen, for their formation [13]. Spherical cancer models, or spheroids, act as intermediates between in vitro cultures of immortalized cancer cells and tumors found in vivo. Spheroids are spontaneously aggregated 3-dimensional (3D) cell clusters that mimic the tumor microenvironment and are useful for evaluating the efficacy and toxicity of anticancer drugs [14].
Human cerebral organoids have provided valuable insight into the early stages of neurodevelopment and disease progression and enable the ability to manipulate these processes in vitro (Fig. 1). A notable example of the application of this technology is the link between circulating Zika virus (ZIKV) and the rise in microcephaly cases in Brazil [5,15]. By exposing human iPSC-derived brain organoids to a specific Brazilian ZIKV strain from an infected patient, researchers can illustrate how the virus targets neural progenitor cells through uniquely expressed membrane receptors [16]. ZIKV infection in neural progenitor cells leads to cell death and cortical plate defects by reducing certain populations of cortical neurons in various layers of the organoid and mouse brain. This organoid model was subsequently used to demonstrate that previously approved medications can prevent infection or inhibit ZIKV replication by blocking its vertical transmission [17,18]. Additionally, organoids can be cultured for several months or even years, affecting their cellular composition and developmental stages.
One key characteristic of the human brain is the development of the outer subventricular zone, which contains many outer radial glial progenitor cells that influence the size and complexity of the human cortical surface. This structure is scarce in the mouse brain, making it an important aspect of organoid research [19]. By introducing variations in the differentiation process, cerebral organoids can generate different types of brain cells, such as those found in the cortex, cerebellum, and thalamus [20,21]. Various methods have been used to create brain organoids using Clustered Regularly Interspaced Short Palindromic Repeats/Cas9 (CRISPR/Cas9) technology, a prominent gene-editing method. This technique enables the manipulation of specific genes to model defective tissues using iPSCs or embryonic stem cells [22,23]. The patch-clamp method, which involves connecting electrodes to form an electrical circuit, has facilitated the understanding and application of the electrophysiological behavior of organoids [24]. This method is particularly useful when combined with light-activated proteins, such as opsins, to create more precise brain organoids [25]. Assembly, a notable approach for the broader application of 3D structured organoids, was introduced in 2017. Assembly combines 2 or more organoids to represent different organs or parts of the brain, offering the advantage of longer survival times [26].
Organoids are primarily used to model various genetic diseases, starting with germline mutations. Research on organoids has extended beyond infectious diseases to include cancer-related and environmental factors (Table 1).
Primary microcephaly is a neurodevelopmental disorder characterized by a reduction in brain size accompanied by neurological symptoms. Although studies using mouse models have investigated its pathophysiology, they have not successfully replicated the disease. Consequently, research has shifted to the use of organoids to better understand and study microcephaly. In patients with primary microcephaly, mutations in the CDK5 regulatory subunit-associated protein 2 (CDK5RAP2) gene have been identified, resulting in loss of the CDK5RAP2 protein. To investigate this, CDK5RAP2 mutations were induced in the organoids by co-electroporation with the GFP and CDK5RAP2 genes [27]. Following a study in 2014, scientists continued to explore the roles of various genes in microcephaly using organoids. Studies have demonstrated that modifying genes, such as WDR6, NARS1, KATNB1, and PTEN, in organoids can induce microcephaly. These findings indicate that mutations in or knockdown of these genes result in phenotypes similar to those observed in microcephaly [28-31].
Sandhoff’s disease is a neurodegenerative disorder characterized by the progressive deterioration of neurons in the brain and spinal cord. Studies using organoids have elucidated a critical role of the HEXB gene in its pathogenesis [32]. Mutations in the LIS1 and YWHAE genes have been identified as major contributing factors to Miller-Dieker syndrome, exemplified by lissencephaly [22]. Owing to the failure of neural cells to migrate to their correct positions within the brain, which results in abnormal localization around the ventricles, periventricular heterotopia has been linked to mutations or knockdowns in the DCHS1 or FAT1 genes as demonstrated by research using organoid models [33].
Research on microcephaly conducted at approximately the same time as studies of ZIKV infection revealed that the latter encountered during the early stages of organoid development triggers apoptosis. This study also identified how ZIKV infection impedes organoid growth, leading to microcephaly [16,34]. During the coronavirus disease 2019 pandemic, organoid models have been employed to study the effects of sudden acute respiratory syndrome coronavirus 2 on the brain and provide clear insight into its effects on neural cells [35,36].
Dysregulation of the DSCAM/PAK1 pathway significantly affects Down syndrome, which results from trisomy of chromosome 21 [37]. In DiGeorge syndrome, which is caused by a 22q11.2 deletion, organoid studies revealed a calcium deficit that affects calcium channels [38]. Neurofibromatosis type 1 is associated with a 17q11.2 microdeletion, and organoid studies have confirmed that this leads to impaired expression of cytokine receptor-like factor 3 and disrupted RhoA signaling [39].
Mosaic diseases are characterized by the presence of different genetic mutations in various cell types within the same individual. Organoid models have also been used to study these diseases and provide valuable insight into their underlying mechanisms. For tuberous sclerosis complex (TSC), studies investigating the role of TSC astrocytes, organoid models were employed. This study revealed that TSC significantly affects astrocyte function in the brain [40]. While some studies using iPSCs to treat Rett syndrome, others utilized organoids. One study demonstrated abnormal electrical activity in organoids, highlighting their potential contribution to epilepsy research [41]. Organoid research initially focused extensively on microcephaly. However, a subsequent study explored macrocephaly using organoids and revealed that the RAB39b-PI3K-mTOR pathway plays a significant role in macrocephaly [42].
Autism spectrum disorder (ASD) is a condition influenced by early developmental processes; its pathogenesis remains complex and is not fully explained by a single mechanism. Organoids have been used extensively to study ASD, including studies using iPSCs derived from patients with autism. Studies have also examined organoids with single gene mutations. Organoid studies identified a key interaction between RAB39b and PI3K components to accelerate mammalian target of rapamycin signaling, which alters cortical neurogenesis and can cause symptoms such as macrocephaly and ASD [42]. Conversely, variations in the AUT2 gene can cause symptoms resembling ASD and microcephaly [43]. Independent of its association with macrocephaly or microcephaly, organoid research revealed that ACTL6B protein deficiencies can cause recessive forms of ASD [4]. Genetic mutations associated with ASD have also been analyzed, with studies showing that organoids derived from patients with ASD overproduce gamma-aminobutyric acid-ergic (GABAergic) inhibitory neurons in idiopathic cases and an association between FOXG1 expression and symptom severity [44,45].
Advancements in molecular specificity owing to DNA methylation have been highly encouraging for elucidating central nervous system tumor biology. This progress led to the identification of genetic alterations affecting tumors, such as gliomas, medulloblastomas, and ependymomas [46,47]. Based on these insights, organoid research has been extensively applied in tumor studies. Recent developments include the creation of organoids for medulloblastomas and high-grade gliomas using methods such as transplantation into immunodeficient mice to establish more stable and prolonged research environments [48]. Various approaches have been employed, including the use of organoids derived from normal brain cells, cells directly obtained from patients with glioma, and genetic modification of human pluripotent stem cells during organoid development [49]. For instance, studies of epidermal growth factor receptor (EGFR) mutations, particularly EGFR variant type III commonly found in glioblastoma multiforme, have demonstrated that temozolomide can induce cell death in organoids [50]. Moreover, organoid research confirmed that mesenchyme homeobox 2 acts as an oncogenic transcriptional regulator in glioblastoma multiforme [51]. Additionally, organoids have achieved an 87% reproduction rate for low-grade gliomas, which are notably challenging to characterize compared with high-grade gliomas [52]. In the context of medulloblastoma, organoid studies have revealed that Otx2 and c-MYC act as inducers and that the EZH2 inhibitor tazemetostat reduces tumorigenesis [53]. In addition, organoid studies revealed that atypical teratoid rhabdoid tumors, which are associated with SMARCB1 inactivation, disrupt neuronal differentiation by impeding maturation [54].
Similar to the expansion of Rett syndrome research in epilepsy studies, organoids have been used in epilepsy research. Epilepsy is both characterized by variable responses to medications and genetically influenced. Organoids have been employed in epilepsy research because of their ability to recreate GABAergic and glutamatergic neurons, thus providing valuable insight into the disease [55]. For STXBP1-related epileptic encephalopathy, organoid studies revealed that neurodegeneration in this encephalopathy is associated with reduced SYNTAXIN1 expression. The emergence of STXBP1-null human endothelial stem cells (hESC) lines leads to severe neurodegeneration [56].
Organoids have been utilized in various research fields beyond genetic influences. Research has advanced our understanding of the effects of organoids on human primary cortical tissue and improved strategies for enhancing treatment efficacy for premature infants [44,57]. Additionally, organoids are instrumental in studying brain development under the influence of toxic substances, such as methadone and valproic acid [58,59]. Furthermore, organoid-based research has provided valuable insights into traumatic and foundational information on neuronal damage and loss [60].
Despite their current limitations, organoid research is advancing rapidly and has demonstrated the potential for application across a wide range of fields. One of the main drawbacks of organoids is their variability even when experiments are conducted under the same conditions, which limits their reproducibility. Research is underway to address this issue by stabilizing organoid cultures, optimizing specific growth conditions, and developing standardized protocols for reproducibility [61]. With the stabilization of culture conditions, human brain organoids have achieved up to 95% cellular differentiation, which is comparable to brain development [62]. Efforts to standardize the organoid experimental environment are ongoing, including the use of polycopolymers to create microfilament-engineered cerebral organoids that form the neuroectoderm [63] and bioreactors with spinning techniques to maintain stable cultures for over a year [64].
Another critical issue with organoids is their inability to generate vasculature, which is essential for providing adequate nutrients for further growth. Currently, organoids lack sufficient vascular structures, and efforts are ongoing to address this limitation [65]. Recent studies have demonstrated the successful creation of human mouse blood vessels using human umbilical vein endothelial cells (human endothelial stem cells or human induced pluripotent stem cells) in organoids with effective function for more than 200 days [66]. Methods have been developed to create and fuse separate brain and blood vessel organoids, enabling the formation of brain-blood barrier–like structures [67].
Additionally, engineering organoids to express human E26 transformation-specific transcription variant 2 has facilitated the development of perfused blood vessels in vivo [68]. These technological advancements have enhanced the reliability and accuracy of organoid research and play a crucial role in disease modeling and drug development. The continued development of new technologies and methodologies is expected to expand the application of organoid research in pediatric patients with neurological illnesses.
Organoid research is emerging as a valuable tool to overcome the limitations of traditional studies, particularly in the field of pediatric neurological disorders. By employing organoids to model a range of conditions, including microcephaly, genetic disorders, epilepsy, and brain tumors, significant insights into the pathophysiology of these diseases have been obtained. Recent technological advancements have enabled precise and repeatable experiments. Organoid research is expected to deepen our understanding of pediatric neurological disorders and contribute to the development of novel therapeutic approaches.
Footnotes
Fig. 1.
Reprogramming of iPSCs and differentiation into cerebral organoids. Peripheral blood (3 mL) is collected and processed to isolate PBMCs. The isolated PBMCs are reprogrammed to generate iPSCs using appropriate reprogramming factors (OCT4, SOX2, KLF4, and c-MYC). Next, the iPSCs are cultured under specific conditions to form embryoid bodies, 3-dimensional aggregates that differentiate into various cell types. The embryoid bodies are further differentiated into cerebral organoids, 3-dimensional brain-like structures that model human brain development and function. PSC, induced pluripotent stem cell; iPSC, induced PSC; PBMCs, peripheral blood mononuclear cells.
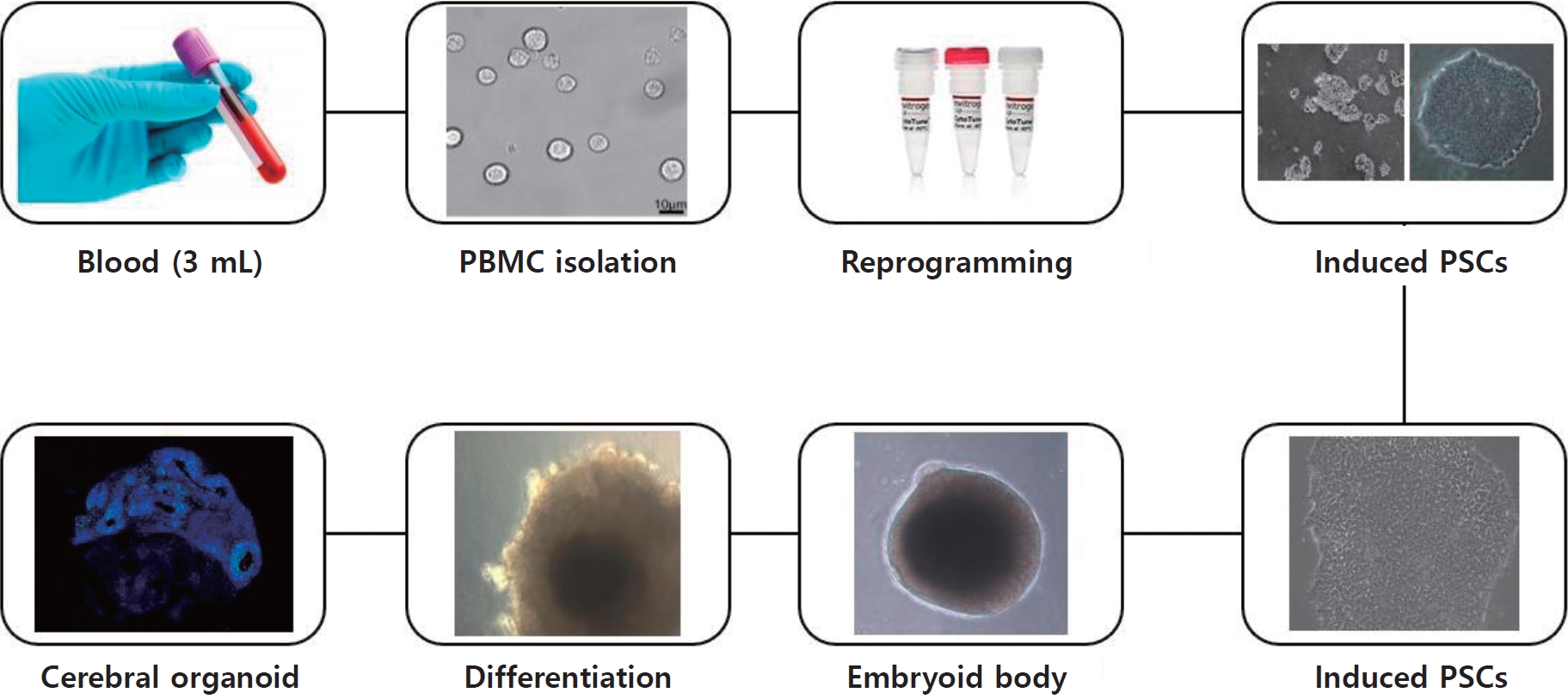
Table 1.
Essential findings of cerebral organoid research on pediatric neurological disorders
ZIKV, Zika virus; COVID-19, coronavirus disease 2019; SARS-CoV-2, sudden acute respiratory syndrome coronavirus 2; VGAT+, vesicular γ-aminobutyric acid (GABA) transporter; NF1, neurofibromatosis type 1; NPCs, neural progenitor cells; ASD, autism spectrum disorder; 3D, 3-dimensional; GABAergic, gamma-aminobutyric acidergic; hiPSCs, human induced pluripotent stem cells; ATRT, atypical teratoid rhabdoid tumor; hPSC, human pluripotent stem cells.
References
1. Durbin MD, Cadar AG, Chun YW, Hong CC. Investigating pediatric disorders with induced pluripotent stem cells. Pediatr Res 2018;84:499–508.




2. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–76.


3. Maherali N, Sridharan R, Xie W, Utikal J, Eminli S, Arnold K, et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell 2007;1:55–70.


4. Wenderski W, Wang L, Krokhotin A, Walsh JJ, Li H, Shoji H, et al. Loss of the neural-specific BAF subunit ACTL6B relieves repression of early response genes and causes recessive autism. Proc Natl Acad Sci U S A 2020;117:10055–66.



5. Mlakar J, Korva M, Tul N, Popović M, Poljšak-Prijatelj M, Mraz J, et al. Zika virus associated with microcephaly. N Engl J Med 2016;374:951–8.


6. Patitucci TN, Ebert AD. SMN deficiency does not induce oxidative stress in SMA iPSC-derived astrocytes or motor neurons. Hum Mol Genet 2016;25:514–23.


7. Corti S, Nizzardo M, Simone C, Falcone M, Nardini M, Ronchi D, et al. Genetic correction of human induced pluripotent stem cells from patients with spinal muscular atrophy. Sci Transl Med 2012;4:165ra162.



8. Yoon KJ, Nguyen HN, Ursini G, Zhang F, Kim NS, Wen Z, et al. Modeling a genetic risk for schizophrenia in iPSCs and mice reveals neural stem cell deficits associated with adherens junctions and polarity. Cell Stem Cell 2014;15:79–91.



9. Wen Z, Nguyen HN, Guo Z, Lalli MA, Wang X, Su Y, et al. Synaptic dysregulation in a human iPS cell model of mental disorders. Nature 2014;515:414–8.




10. Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature 2011;473:221–5.




11. Madison JM, Zhou F, Nigam A, Hussain A, Barker DD, Nehme R, et al. Characterization of bipolar disorder patient-specific induced pluripotent stem cells from a family reveals neurodevelopmental and mRNA expression abnormalities. Mol Psychiatry 2015;20:703–17.




12. Mellios N, Feldman DA, Sheridan SD, Ip JPK, Kwok S, Amoah SK, et al. MeCP2-regulated miRNAs control early human neurogenesis through differential effects on ERK and AKT signaling. Mol Psychiatry 2018;23:1051–65.




13. Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science 2014;345:1247125.


14. Elkabetz Y, Panagiotakos G, Al Shamy G, Socci ND, Tabar V, Studer L. Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage. Genes Dev 2008;22:152–65.



15. Campos GS, Bandeira AC, Sardi SI. Zika virus outbreak, Bahia, Brazil. Emerg Infect Dis 2015;21:1885–6.



16. Cugola FR, Fernandes IR, Russo FB, Freitas BC, Dias JL, Guimarães KP, et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature 2016;534:267–71.




17. Shiryaev SA, Mesci P, Pinto A, Fernandes I, Sheets N, Shresta S, et al. Repurposing of the anti-malaria drug chloroquine for Zika virus treatment and prophylaxis. Sci Rep 2017;7:15771.




18. Mesci P, Macia A, Moore SM, Shiryaev SA, Pinto A, Huang CT, et al. Blocking Zika virus vertical transmission. Sci Rep 2018;8:1218.




19. Wang X, Tsai JW, LaMonica B, Kriegstein AR. A new subtype of progenitor cell in the mouse embryonic neocortex. Nat Neurosci 2011;14:555–61.




20. Su HL, Muguruma K, Matsuo-Takasaki M, Kengaku M, Watanabe K, Sasai Y. Generation of cerebellar neuron precursors from embryonic stem cells. Dev Biol 2006;290:287–96.


21. Panoutsopoulos AA. Organoids, assembloids, and novel biotechnology: steps forward in developmental and disease-related neuroscience. Neuroscientist 2021;27:463–72.



22. Iefremova V, Manikakis G, Krefft O, Jabali A, Weynans K, Wilkens R, et al. An organoid-based model of cortical development identifies non-cell-autonomous defects in Wnt signaling contributing to Miller-Dieker Syndrome. Cell Rep 2017;19:50–9.


23. Musunuru K. Genome editing of human pluripotent stem cells to generate human cellular disease models. Dis Model Mech 2013;6:896–904.




24. Quadrato G, Nguyen T, Macosko EZ, Sherwood JL, Min Yang S, Berger DR, et al. Cell diversity and network dynamics in photosensitive human brain organoids. Nature 2017;545:48–53.




25. Moore AK, Wehr M. Parvalbumin-expressing inhibitory interneurons in auditory cortex are well-tuned for frequency. J Neurosci 2013;33:13713–23.



26. Marton RM, Pașca SP. Organoid and assembloid technologies for investigating cellular crosstalk in human brain development and disease. Trends Cell Biol 2020;30:133–43.


27. Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, et al. Cerebral organoids model human brain development and microcephaly. Nature 2013;501:373–9.




28. Zhang W, Yang SL, Yang M, Herrlinger S, Shao Q, Collar JL, et al. Modeling microcephaly with cerebral organoids reveals a WDR62-CEP170-KIF2A pathway promoting cilium disassembly in neural progenitors. Nat Commun 2019;10:2612.




29. Wang L, Li Z, Sievert D, Smith DEC, Mendes MI, Chen DY, et al. Loss of NARS1 impairs progenitor proliferation in cortical brain organoids and leads to microcephaly. Nat Commun 2020;11:4038.




30. Jin M, Pomp O, Shinoda T, Toba S, Torisawa T, Furuta K, et al. Katanin p80, NuMA and cytoplasmic dynein cooperate to control microtubule dynamics. Sci Rep 2017;7:39902.




31. Dhaliwal N, Choi WWY, Muffat J, Li Y. Modeling PTEN overexpression-induced microcephaly in human brain organoids. Mol Brain 2021;14:131.




32. Allende ML, Cook EK, Larman BC, Nugent A, Brady JM, Golebiowski D, et al. Cerebral organoids derived from Sandhoff disease-induced pluripotent stem cells exhibit impaired neurodifferentiation. J Lipid Res 2018;59:550–63.



33. Klaus J, Kanton S, Kyrousi C, Ayo-Martin AC, Di Giaimo R, Riesenberg S, et al. Altered neuronal migratory trajectories in human cerebral organoids derived from individuals with neuronal heterotopia. Nat Med 2019;25:561–8.



34. Li Y, Muffat J, Omer A, Bosch I, Lancaster MA, Sur M, et al. Induction of expansion and folding in human cerebral organoids. Cell Stem Cell 2017;20:385–96.e3.



35. Pellegrini L, Albecka A, Mallery DL, Kellner MJ, Paul D, Carter AP, et al. SARS-CoV-2 infects the brain choroid plexus and disrupts the blood-CSF barrier in human brain organoids. Cell Stem Cell 2020;27:951–61.e5.



36. Carroll JA, Foliaki ST, Haigh CL. A 3D cell culture approach for studying neuroinflammation. J Neurosci Methods 2021;358:109201.



37. Tang XY, Xu L, Wang J, Hong Y, Wang Y, Zhu Q, et al. DSCAM/PAK1 pathway suppression reverses neurogenesis deficits in iPSC-derived cerebral organoids from patients with Down syndrome. J Clin Invest 2021;131:e135763.



38. Khan TA, Revah O, Gordon A, Yoon SJ, Krawisz AK, Goold C, et al. Neuronal defects in a human cellular model of 22q11.2 deletion syndrome. Nat Med 2020;26:1888–98.




39. Wegscheid ML, Anastasaki C, Hartigan KA, Cobb OM, Papke JB, Traber JN, et al. Patient-derived iPSC-cerebral organoid modeling of the 17q11.2 microdeletion syndrome establishes CRLF3 as a critical regulator of neurogenesis. Cell Rep 2021;36:109315.



40. Dooves S, van Velthoven AJH, Suciati LG, Heine VM. Neuron-glia interactions in tuberous sclerosis complex affect the synaptic balance in 2D and organoid cultures. Cells 2021;10:134.



41. Samarasinghe RA, Miranda OA, Buth JE, Mitchell S, Ferando I, Watanabe M, et al. Identification of neural oscillations and epileptiform changes in human brain organoids. Nat Neurosci 2021;24:1488–500.




42. Zhang W, Ma L, Yang M, Shao Q, Xu J, Lu Z, et al. Cerebral organoid and mouse models reveal a RAB39b-PI3K-mTOR pathway-dependent dysregulation of cortical development leading to macrocephaly/autism phenotypes. Genes Dev 2020;34:580–97.



43. Fair SR, Schwind W, Julian DL, Biel A, Guo G, Rutherford R, et al. Cerebral organoids containing an AUTS2 missense variant model microcephaly. Brain 2023;146:387–404.




44. Paulsen B, Velasco S, Kedaigle AJ, Pigoni M, Quadrato G, Deo AJ, et al. Autism genes converge on asynchronous development of shared neuron classes. Nature 2022;602:268–73.


45. Mariani J, Coppola G, Zhang P, Abyzov A, Provini L, Tomasini L, et al. FOXG1-dependent dysregulation of GABA/glutamate neuron differentiation in autism spectrum disorders. Cell 2015;162:375–90.



47. Kumar R, Liu APY, Orr BA, Northcott PA, Robinson GW. Advances in the classification of pediatric brain tumors through DNA methylation profiling: from research tool to frontline diagnostic. Cancer 2018;124:4168–80.




48. Lago C, Gianesello M, Santomaso L, Leva G, Ballabio C, Anderle M, et al. Medulloblastoma and high-grade glioma organoids for drug screening, lineage tracing, co-culture and in vivo assay. Nat Protoc 2023;18:2143–80.



49. Wen J, Liu F, Cheng Q, Weygant N, Liang X, Fan F, et al. Applications of organoid technology to brain tumors. CNS Neurosci Ther 2023;29:2725–43.



50. Kim HM, Lee SH, Lim J, Yoo J, Hwang DY. The epidermal growth factor receptor variant type III mutation frequently found in gliomas induces astrogenesis in human cerebral organoids. Cell Prolif 2021;54:e12965.




51. Schönrock A, Heinzelmann E, Steffl B, Demirdizen E, Narayanan A, Krunic D, et al. MEOX2 homeobox gene promotes growth of malignant gliomas. Neuro Oncol 2022;24:1911–24.




52. Abdullah KG, Bird CE, Buehler JD, Gattie LC, Savani MR, Sternisha AC, et al. Establishment of patient-derived organoid models of lower-grade glioma. Neuro Oncol 2022;24:612–23.




53. Ballabio C, Anderle M, Gianesello M, Lago C, Miele E, Cardano M, et al. Modeling medulloblastoma in vivo and with human cerebellar organoids. Nat Commun 2020;11:583.




54. Parisian AD, Koga T, Miki S, Johann PD, Kool M, Crawford JR, et al. SMARCB1 loss interacts with neuronal differentiation state to block maturation and impact cell stability. Genes Dev 2020;34:1316–29.



56. Patzke C, Südhof TC. The conditional KO approach: Cre/Lox technology in human neurons. Rare Dis 2016;4:e1131884.



57. Pașca AM, Park JY, Shin HW, Qi Q, Revah O, Krasnoff R, et al. Human 3D cellular model of hypoxic brain injury of prematurity. Nat Med 2019;25:784–91.




58. Yao H, Wu W, Cerf I, Zhao HW, Wang J, Negraes PD, et al. Methadone interrupts neural growth and function in human cortical organoids. Stem Cell Res 2020;49:102065.


59. Meng Q, Zhang W, Wang X, Jiao C, Xu S, Liu C, et al. Human forebrain organoids reveal connections between valproic acid exposure and autism risk. Transl Psychiatry 2022;12:130.




60. Ramirez S, Mukherjee A, Sepulveda S, Becerra-Calixto A, Bravo-Vasquez N, Gherardelli C, et al. Modeling traumatic brain injury in human cerebral organoids. Cells 2021;10:2683.



61. Englund C, Fink A, Lau C, Pham D, Daza RA, Bulfone A, et al. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J Neurosci 2005;25:247–51.



62. Velasco S, Kedaigle AJ, Simmons SK, Nash A, Rocha M, Quadrato G, et al. Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature 2019;570:523–7.




63. Lancaster MA, Corsini NS, Wolfinger S, Gustafson EH, Phillips AW, Burkard TR, et al. Guided self-organization and cortical plate formation in human brain organoids. Nat Biotechnol 2017;35:659–66.




64. Lancaster MA, Knoblich JA. Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc 2014;9:2329–40.




66. Shi Y, Sun L, Wang M, Liu J, Zhong S, Li R, et al. Vascularized human cortical organoids (vOrganoids) model cortical development in vivo. PLoS Biol 2020;18:e3000705.








 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


