Article Contents
| Clin Exp Pediatr > Volume 68(8); 2025 |
|
Abstract
Background
Purpose
Methods
Footnotes
Fig. 2.
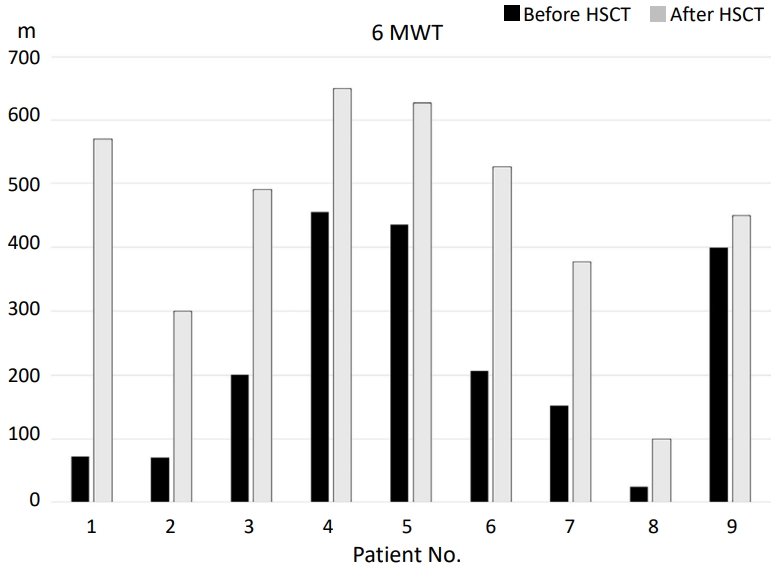
Table 1.
Values are presented as median (range) or number (%).
HSCT, hematopoetic stem cell transplantation; Tx, transplantation; ERT, enzyme replacement therapy; MSD, matched sibling donor; MUD, matched unrelated donor; HLA, human leukocyte antigen; Flu, fludarabine; TT, thiotepa; Treo, treosulfan; GVHD, graft-versus-host disease; Tacr, tacrolimus; Mtx, methotrexate; CsA, cyclosporine A; BM, bone marrow; PBSC, peripheral blood stem cell.
Table 2.
| Characteristic | Value |
|---|---|
| Engraftment (day) | |
| Neutrophil | 14 (11–20) |
| Platelet | 11 (7–15) |
| Chimerism (%) | |
| First (4 wk after HSCT) | 99 (86–100) |
| Last (at the last follow-up) | 98 (78–100) |
| Acute GVHD grades 2–4 | |
| 100 Days | 4 (24) |
| Acute GVHD grades 3–4 | |
| 100 Days | 1 (6) |
| Chronic GVHD (mild) | 2 (12) |
| Complications | |
| CMV | 6 (35) |
| Engraftment syndrome | 2 (12) |
| Autoimmune | 1 (6) |
| Hemorrhagic cystitis | 1 (6) |
| VOD | 0 (0) |
| Pre-HSCT enzyme levels | 0.1 (0.1–1.0) |
| Post-HSCT enzyme levels | 100 (40–230) |
| Change in 6-MWT after HSCT (n=9)a) | |
| Change (m) | 290 (50–498) |
| Follow-up for 6-MWT (mo) | 9 (6–22) |
| Follow-up (mo) | 14 (9–29) |
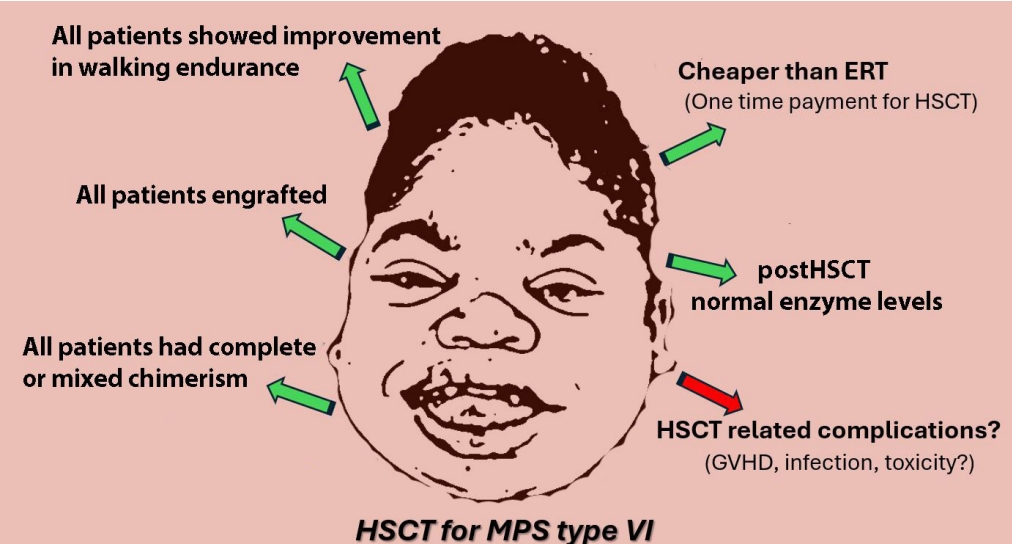
| Survival | 17 (100) |
| DGFSb) | 17 (100) |
Values are presented as median (range) or number (%).
HSCT, hematopoetic stem cell transplantation; GVHD, graft-versus-host disease; CMV, cytomegalovirus; VOD, veno-occlusive disease; 6-MWT, 6-minute walk test; DGFS, disease and GVHD-free survival.
The enzyme level in the first evaluation (median 4th month of HSCT [range, 3–11 months]; [N>10 nmol/mg.h])
Table 3.
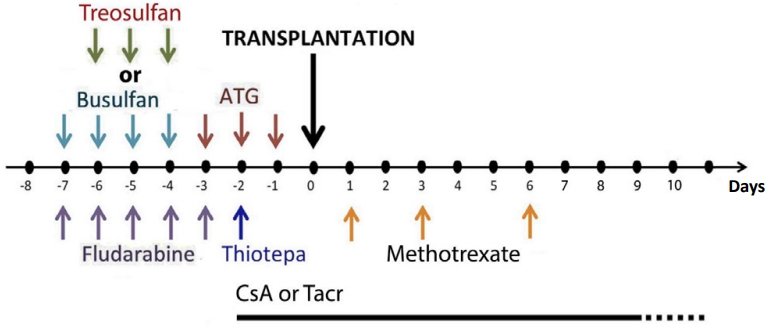
| Patient No./sex/age (yr)a) | ARSB gene mutation | Donor | Stem cell source | Conditioning regimen | ATG | aGVHD | cGVHD | Chimerism (%)b) | Enzyme level | Improvement in post-HSCT 6-MWT (m)/status |
|---|---|---|---|---|---|---|---|---|---|---|
| 1/F/7.2 | c.962 T>C | MUD | PBSC | Flu+Bu | + | Grade 2 | - | 99 | Normal | 498/Alive, No GVHD |
| 2/F/3.5 | c.903 C>G | MSD | PBSC | Flu+Bu | + | - | - | 82 | Normal | -/Alive |
| 3/F/9.8 | c.962 T>C | MUD | PBSC | Flu+Bu+TT | + | - | - | 99 | Normal | -/Alive |
| 4/F/14.3 | c.962 T>C | MSD | BM | Flu+Treo+TT | + | - | - | 100 | Normal | 230/Alive |
| 5/M/13.7 | c.962 T>C | MUD | PBSC | Flu+Bu+TT | + | - | - | 98 | Normal | 290/Alive |
| 6/F/3.5 | c.478 C>T | MUD | BM | Flu+Bu+TT | + | - | - | 100 | Normal | 195/Alive |
| 7/F/10.3 | c.1575delC | MSD | BM | Flu+Bu+TT | + | Grade 2 | Mild cGVHD | 99 | Normal | 191/Alive, No GVHD |
| 8/F/10.9 | c.962 T>C | MUD | BM | Flu+Treo+TT | + | - | - | 100 | Normal | -/Alive |
| 9/F/7.2 | c.962 T>C | MUD | PBSC | Flu+Bu+TT | + | - | - | 89 | Normal | -/Alive |
| 10/F/2.6 | c.962 T>C | MUD | PBSC | Flu+Bu+TT | + | - | - | 78 | Normal | 320/Alive |
| 11/M/1.0 | c.962 T>C | MSD | BM | Flu+Bu+TT | + | - | - | 84 | Normal | -/Alive |
| 12/F/10.9 | c.962 T>C | MUD | PBSC | Flu+Bu | + | Grade 3 | - | 99 | Normal | -/Alive, No GVHD |
| 13/M/5.7 | c.962 T>C | MUD | PBSC | Flu+Bu+TT | + | - | - | 98 | Normal | 225/Alive |
| 14/F/7.4 | c.962 T>C | MUD | PBSC | Flu+Bu | + | Grade 2 | - | 97 | Normal | 75/Alive, No GVHD |
| 15/M/2.8 | c.962 T>C | MUD | PBSC | Flu+Bu+TT | + | - | - | 99 | Normal | 50/Alive |
| 16/M/8.2 | c.962 T>C | MUD | PBSC | Flu+Bu | + | - | Mild cGVHD | 98 | Normal | -/Alive, No GVHD |
| 17/M/15.9 | c.962 T>C | MUD | PBSC | Flu+Bu+TT | + | - | - | 99 | Normal | -/Alive |
ATG, antithymocyte globulin; GVHD, graft-versus-host disease; aGVHD, acute GVHD; cGVHD, chronic GVHD; HSCT, hematopoetic stem cell transplantation; 6-MWT, 6-minute walk test; MUD, matched unrelated donor; MSD, matched sibling donor; PBSC, peripheral blood stem cell; BM, bone marrow; Flu, fludarabine; Bu, busulfan; TT, thiotepa; Treo, treosulfan.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


