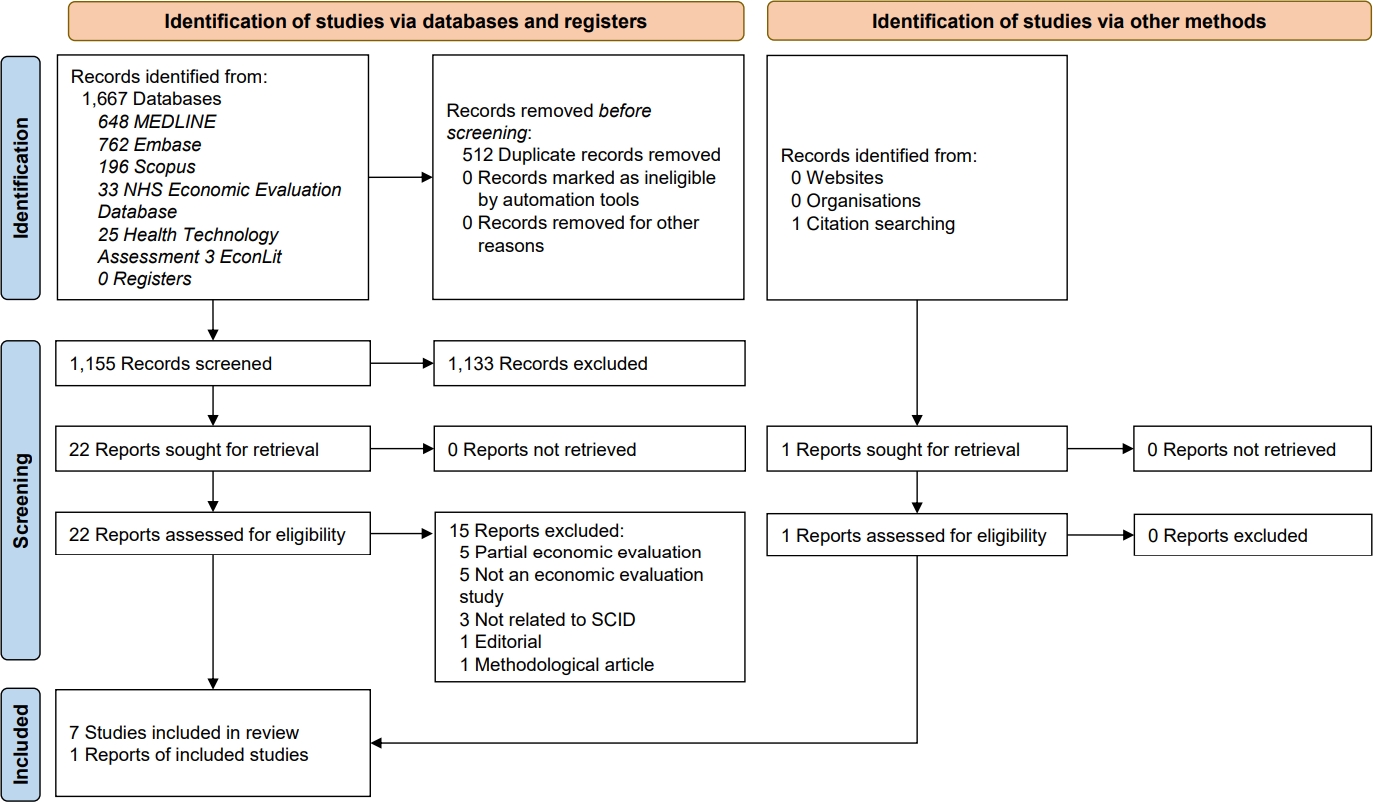
Article Contents
| Clin Exp Pediatr > Volume 68(9); 2025 |
|
Abstract
Supplementary materials
Supplementary Table 1.
Supplementary Table 2.
Footnotes
Table 1.
| Characteristic | No. (%) |
|---|---|
| Country | |
| USA | 2 (25.0) |
| Netherlands | 2 (25.0) |
| Australia | 2 (25.0) |
| UK | 1 (12.5) |
| New Zealand | 1 (12.5) |
| Language, English | 8 (100) |
| Year published | |
| 2008–2017 | 3 (37.5) |
| 2018–2023 | 5 (62.5) |
| Study type | |
| Cost-effectiveness | 2 (25.0) |
| Cost-utility | 6 (75.0) |
| CEA and CUA | 0 (0) |
| Perspective | |
| Societal | 1 (12.5) |
| Health system | 6 (75.0) |
| Health system and societal | 1 (12.5) |
| Time horizona) | |
| 1 Year or less | 0 (0) |
| More than 1 to 5 years | 3 (37.5) |
| More than 5 years to 10 years | 0 (0) |
| More than 10 years to 30 years | 0 (0) |
| More than 30 years to lifetime | 6 (75.0) |
| Not reported | 1 (12.5) |
| Discount rate | |
| No discounting | 1 (12.5) |
| Discounting (3%–3.5%) | 6 (75.0) |
| Other | 1 (12.5) |
| Study design, model-based | 8 (100) |
| Type of modeling | |
| Markov | 3 (37.5) |
| Decision tree | 3 (37.5) |
| Other | 2 (25.0) |
| Outcome | |
| QALY | 5 (62.5) |
| DALY | 0 (0) |
| LY | 2 (25.0) |
| LY and QALY | 1(12.5) |
| Reported cutoff for the TREC excision circles screening test | |
| Yes | 3 (37.5) |
| No | 5 (62.5) |
| Type of cost included | |
| Direct | 7 (87.5) |
| Direct and indirect | 1 (12.5) |
| Sensitivity analysisb) | |
| Probabilistic or 1-way only | 5 (62.5) |
| Deterministic only | 0 (0) |
| Deterministic and probabilistic | 2 (25.0) |
| Not specified | 1 (12.5) |
CEA, cost-effectiveness analysis; CUA, cost-utility analysis; QALY, qualityadjusted life-years; DALY, disability-adjusted life years; LY, life years; TREC, T-cell receptor excision circles.
b) Studies conducted in both probabilistic and deterministic categories are included in the probabilistic and deterministic categories. The study by van den Akker-van Marle et al. [21] did not adequately specify the sensitivity analyses used.
Table 2.
| Study | Currency (yr) | ICER | USD (2022) converted value | Outcome measures |
|---|---|---|---|---|
| Bessey et al. [19] (2019) | Pound (2014/15) | 18,222 | 30,214 | QALY gained |
| Chan et al. [4] (2011) | USD (2005) | 27,907 | 38,146 | QALY gained |
| 25,429 | 34,759 | Life years saved | ||
| Ding et al. [14] (2016) | USD (2015) | 35,311 | 40,267 | Life years saved |
| HPCG et al. [29] (2014) | NZD (2013) | 30,409 | 24,211 | Life years saved |
| Shih et al. [20] (2022) | USD (2021) | 33,600 | 34,295 | QALY gained |
| Shih et al. [24] (2022) | USD (2018) | 33,600 | 34,295 | QALY gained |
| Van den Akker-van Marle et al. [21] (2021)a) | EURO (2020) | 44,300 | 54,282 | QALY gained |
| Van der Ploeg et al. [18] (2019) | EURO (2016) | 33,400 | 46,885 | QALY gained |
ICER, incremental cost-effectiveness ratio; USD, United States dollars; NZD, New Zealand dollars; QALY, quality-adjusted life-years; TREC, T-cell receptor excision circle; HPCG, Health Partners Consulting Group.
The currency was converted based on Shemilt and James. [22] Website link: https://eppi.ioe.ac.uk/costconversion/
Table 3.
| Study | Country | Main objective | Population | Model structure | Outcome measures/ | Time horizon | Discount rate | Currency (yr) | Cutoff for the TREC screening test |
|---|---|---|---|---|---|---|---|---|---|
| Bessey et al. [19] (2019) | UK | To assess the cost-effectiveness of SCID screening for the National Screening Committee | Patients with SCID were identified through screening or those diagnosed early due to a family history. | Decision-tree cost-effectiveness model. | QALYs | Lifetime horizon | 3.5% | British Pound (2014/15) | Baseline: 20 copies/μL |
| N=82 | No screening vs. newborn screening | Sensitivity analysis: 30 copies/μL | |||||||
| Chan et al. [4] (2011) | USA | To evaluate the cost-effectiveness of universal neonatal screening for SCID. | Children with SCID | Markov model analytic decision tree. | Life years and QALYs | 70-Yr time horizon | 3% | USD (2005) | NR |
| No screening vs. newborn screening. | |||||||||
| Gross-costing approach to estimate medical and nonmedical costs for each health state | |||||||||
| Ding et al. [14] (2016) | USA | To evaluate the expected cost-effectiveness and net benefit of the recent newborn screening implementation for SCID. | Children with SCID | Decision tree model. | The number of life-years saved and the cost-benefit analysis version place a dollar value on averted deaths. | The time horizon for assessing outcomes is 5 years. | 3% | NR | NR |
| Lifetime horizon for assessing survival. | |||||||||
| HPCG et al. [29] (2014) | New Zealand | To estimate the incremental cost-effectiveness per life-year gained if newborn testing for SCID was introduced. | Children with SCID | A hypothetical cohort of children moves through the decision tree based on a predetermined transition probabilities | Survival years (life-years) | Lifetime horizon | 3.5% | NZD | NR |
| Shih et al. [20] (2022) | Australia | To model the cost-effectiveness of pilot population-based NBS for SCID from the government perspective in Australia. | Children with SCID | Markov cohort simulations were nested within an analytic decision model. | QALY | The time horizon of 5 and 60 yr | 3% | USD | NR |
| Early diagnosis of SCID by NBS vs late diagnosis of SCID by clinical symptoms without NBS | |||||||||
| Shih et al. [24] (2022) | Australia | To examine the costs and outcomes associated with the introduction of universal NBS for SCID | Newborns with SCID | A decision analytical model nested with Markov simulations | QALY | 5- and 60-year time horizon | 3% | USD (2018) | NR |
| Van den Akker-van Marle et al. [21] (2021) | Netherlands | To calculate the cost of different NBS strategies for SCID using real-life data | Newborns obtained a positive screening result for SCID. | Prospective implementation study | Cost of testing per child for SCID, diagnostics after screen positive results, cost-effectiveness ratios. | NR | NR | EURO (2020) | TREC: ≤6 copies/μL, ≤10 copies/μL |
| N=6 | |||||||||
| Van der Ploeg et al. [18] (2019) | Netherlands | To compare lifetime costs and effects of NBS for SCID with a situation without screening in the Netherlands by extending a previously developed deterministic decision analysis model | Children with SCID | Deterministic decision analysis model | Number of deaths due to SCID per 100,000 children, quality-adjusted life years, gained total yearly healthcare costs. | Lifetime | 3% | EURO (2016) | TREC: <25copies/μL a cutoff value of <20, 0.20% of all children needed a retest. |
NBS, newborn blood spot screening; SCID, severe combined immunodeficiency; TREC, T-cell receptor excision circles; QALY, quality-adjusted life years; USD, US dollars; NR, not reported; HPCG, Health Partners Consulting Group.
The included studies investigated NBS for SCID using TREC based on the core 2010 US Recommended Uniform Screening panel suggestions.
Table 4.
| Study | Perspective | Main conclusion | Key findings (ICER) | Source of uncertainty |
|---|---|---|---|---|
| Bessey et al. [19] (2019) | NHS and personal social services perspective | Reduction in SCID mortality from 8.1 (5–12) to 1.7 (0.6–4.0) cases per year of screening. | The ICER for SCID screening is £18,222 (£12,013, £27,763) per QALY gained, with 65% and 99% probability of this being considered cost-effectiveness at a threshold of £20,000 and £30,000, respectively. | Change in the mortality rates |
| False positives | ||||
| The identification of children with non-SCID TCL | ||||
| Chan et al. [4] (2011) | Societal perspective | Screening costs $4.22/infant, and universal screening for SCID would be a cost-effective means to improve the quality and duration of life for children with SCID. | ICER ($ / life year saved) $25,429 | Incidence of disease |
| ICER ($/QALY saved) $27,907 | Specificity of the screening test | |||
| Universal screening in the USA would cost approximately $22.4 million/yr with a gain of 880 life years and 802 QALYs | Screening (vs no screening) is dominant and cost-saving in all scenarios where WTP is less than $50,000/QALY. | |||
| Ding et al. [14] (2016) | Healthcare system perspective | The midpoint value of a statistical life of $9 million, the estimated net benefit of $3.19 million for a single year’s Washington birth cohort, results from subtracting costs from a total benefit of $3.94 million. | ICER ($/life year saved) $35,311 | Relative treatment costs of late-diagnosed vs. early-diagnosed cases |
| Newborn screening for SCID in Washington state (USA) is likely to be cost-effective and to show positive net economic benefit. | ||||
| HPCG et al. [29] (2014) | Healthcare system perspective | Incorporating SCID screening into New Zealand’s newborn metabolic screening program seems to align with established healthcare interventions. | ICER (NZD/life years gained) NZD 30,409. | Incidence rate |
| Adding NBS for SCID to the NMSP, inclusive of treatment | HSCT treatment cost | |||
| Costs would be around $460,000 per year. | Discount rate scenarios | |||
| Shih et al. [20] (2022) | Healthcare system perspective | In the Australian context, screening for SCID should be introduced into the current NBS program from both clinical and economic perspectives. | ICER ($/QALY saved) US$33,600 | SCID incidence |
| Probabilistic sensitivity analysis showed that more than half of the simulated ICERs were considered cost-effective against the common willingness-to-pay threshold of A$50,000/QALY (US$35,000/QALY). | Discount rate | |||
| Shih et al. [24] (2022) | Healthcare system perspective | The introduction of NBS for SCID provides good value for money from the long-term clinical and economic perspective. | Screening each infant in the population for newborn-related costs and QALYs led to an ICER of $144,000 per QALY over a 5-year period. Looking at a span of 60 years (from the governmental standpoint), this approach would result in an ICER of $33,600 per QALY. | SCID incidence |
| The cost of the new disease-modifying therapies | ||||
| Discount rate | ||||
| Van den Akker-van Marle et al. [21] (2021) | Healthcare system perspective | From an economic perspective, strategies with a lower number of referrals, such as distinguishing urgent versus less urgent referrals, are more advantageous. | Cost-effectiveness ratios varied from EURO 41,300 per QALY for the screening strategy with TREC ≤6 copies/punch to EURO 44,100 for the screening strategy with a cutoff value of TREC ≤10 copies/punch. | The small-scale nature of pilot studies and the low referral rates |
| The costs and effects of the new screening algorithm were partly based on assumptions. | ||||
| Van der Ploeg et al. [18] (2019) | Healthcare system perspective | Although there is still much ambiguity surrounding the cost-effectiveness estimate, NBS for SCID may be cost-effective. | NBS for SCID is expected to reduce mortality from 0.57 to 0.23 per 100,000 children at additional healthcare costs of €390,800. | The impact of false-positive screening results was not considered. |
| The cost-utility ratio is €33,400 per QALY gained. |
ICER, incremental cost-effectiveness ratio; NHS, National Health Service; SCID, severe combined immunodeficiency; QALY, quality-adjusted life years; TCR, T-cell receptor; WTP, willingness to pay; NMSP, newborn metabolic screening programme; NBS, newborn blood spot screening; HSCT, hematopoietic stem cell transplant; TREC, T-cell receptor excision circles.
Table 5.
| No. | Topic | Bessey et al. [19] (2019) | Chan et al. [4] (2011) | Ding et al. [14] (2016) | HPCG et al. [29] (2014) | Shih et al. [24] (2022) | Shih et al. [20] (2022) | Van den Akker-van Marle et al. [21] (2021) | Van der Ploeg et al. [18] (2019) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Title | Y | Y | Y | Y | Y | Y | Y | Y |
| 2 | Abstract | Y | Y | Y | Y | Y | Y | Y | Y |
| Introduction | |||||||||
| 3 | Background and objectives Methods | Y | Y | Y | Y | Y | Y | Y | Y |
| 4 | Health economic analysis plan | Y | Y | Y | Y | P | Y | Y | Y |
| 5 | Study population | Y | Y | Y | Y | Y | Y | Y | Y |
| 6 | Setting and location | Y | Y | Y | Y | Y | Y | Y | Y |
| 7 | Comparators | Y | Y | Y | Y | Y | Y | Y | Y |
| 8 | Perspective | Y | Y | Y | Y | Y | Y | Y | Y |
| 9 | Time horizon | Y | Y | Y | Y | Y | Y | Y | Y |
| 10 | Discount rate | Y | Y | Y | Y | Y | Y | Y | Y |
| 11 | Selection of outcomes | Y | Y | Y | P | Y | Y | Y | Y |
| 12 | Measurement of outcomes | Y | Y | Y | P | Y | Y | Y | Y |
| 13 | Valuation of outcomes | NR | Y | P | P | P | NR | Y | Y |
| 14 | Measurement and valuation of resources and costs | NR | Y | Y | Y | P | Y | Y | Y |
| 15 | Currency, price date, and conversion | P | P | Y | Y | P | Y | Y | Y |
| 16 | Rationale and description of the model | Y | Y | Y | Y | Y | Y | Y | Y |
| 17 | Analytics and assumptions | NR | Y | Y | Y | Y | Y | Y | P |
| 18 | Characterizing heterogeneity | P | P | Y | NR | P | Y | Y | P |
| 19 | Characterizing distributional effects | NR | NR | NR | NR | NR | NR | NR | NR |
| Results | |||||||||
| 22 | Study parameters | Y | P | P | Y | Y | P | P | Y |
| 23 | Summary of main results | Y | Y | Y | Y | Y | Y | Y | Y |
| 24 | Effect of uncertainty | Y | Y | Y | Y | Y | Y | Y | Y |
| 25 | Effect of engagement with patients and others affected by the study | NR | NR | P | P | P | P | NR | NR |
| Discussion | |||||||||
| 26 | Study findings, limitations, generalizability, and current knowledge | P | Y | P | P | P | Y | P | P |
| Other relevant information | |||||||||
| 27 | Source of funding | Y | Y | Y | NR | Y | Y | Y | Y |
| 28 | Conflicts of interest | Y | Y | Y | NR | Y | Y | Y | Y |
| Total score | 19.5/26 | 22.5/26 | 23/26 | 19.5/26 | 21.5/26 | 23/26 | 23/26 | 22.5/26 | |
| Percentage (Y + P) | 75% | 86% | 88% | 75% | 83% | 88% | 88% | 86% |
CHEERS, Consolidated Health Economic Evaluation Reporting Standards; HPCG, Health Practitioners Consulting Group; NR, not reported; P, partial (value=0.5); Y, positive (value=1).
Topic 20, “Characterizing uncertainty,” was not used because describing methods to characterize any sources of uncertainty in the analysis was deemed not applicable for the selected studies. Topic 21 in the CHEERS checklist, “Approach to engagement with patients and others affected by the study,” was not used because approaches used to engage patients or service recipients, the general public, communities, or stakeholders (such as clinicians or payers) in the study designed was deemed not applicable.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


