Article Contents
| Korean J Pediatr > Volume 55(6); 2012 |
Abstract
Atopic dermatitis (AD) is an immune disorder that is becoming increasingly prevalent throughout the world. The exact etiology of AD remains unknown, and a cure for AD is not currently available. The hypothesis that appropriate early microbial stimulation contributes to the establishment of a balanced immune system in terms of T helper type Th1, Th2, and regulatory T cell (Treg) responses has led to the use of probiotics for the prevention and treatment of AD in light of various human clinical studies and animal experiments. Meta-analysis data suggests that probiotics can alleviate the symptoms of AD in infants. The effects of balancing Th1/Th2 immunity and enhancing Treg activity via the interaction of probiotics with dendritic cells have been described in vitro and in animal models, although such an effect has not been demonstrated in human studies. In this review, we present some highlights of the immunomodulatory effects of probiotics in humans and animal studies with regard to their effects on the prevention of AD.
Atopic dermatitis (AD) is a common, chronic, and refractory skin disease manifesting as eczema and pruritus with repeated exacerbations and regressions1). The prevalence of allergic diseases such as AD, asthma, and allergic rhinitis (AR) has increased throughout the world during the last 30 years, with the cumulative prevalence of AD in children reaching 8 to 20%2), although the eczema symptom prevalence for children 13 to 14 years of age has decreased in some previously high-prevalence areas in the developed world, such as the United Kingdom and New Zealand3). The prevalence of allergy tends to be lower in a family with a higher number of siblings; the incidence of allergic diseases increases when one moves from a low-prevalence area to a high-prevalence area and is higher in urban areas than in rural areas. These observations emphasize the importance of environmental factors in the development of allergic diseases, as well the role of genetic factors and the maturity of epithelial cell barrier functions4). A systematic review found no strong evidence of a protective effect of exclusive breastfeeding for at least 3 months against eczema, even in those with a positive family history of atopy5). Environmental factors that may contribute to the increased development of AD include decreased microbial exposure due to urbanization, the use of vaccines and antibiotics, and improved infant sanitation. Exposure of infants to farm animals, pets, and daycare environments is known to be helpful for the introduction of benign challenges, including various non-pathogenic microorganisms, which leads to the establishment of protective immunity against allergic disorders4). The intestinal immune system comprises the largest portion of the overall immune system and remains exposed to intestinal bacteria, thus accounting for the largest source of microbial exposure in humans. The immune system of neonatal infants is not fully developed and is prone to an immature T helper type Th2-dominant state. Infants undergo environment-driven maturation to establish a balance between Th1, Th2, Th17, and regulatory T cell (Treg) responses; appropriate microbial stimulation in early life contributes to the establishment of a balanced immune system6). In AD, the onset of acute skin lesions is initiated by Th2-dominant cells. If this initial Th2 cellular response is not suppressed, a subsequent Th1 response is induced, consisting of inflammatory reactions resulting in chronic inflammation due to the secretion of pro-inflammatory cytokines by dying keratinocytes7).
Probiotics administered in sufficient amounts can have a beneficial effect on the health of the host8). Probiotics can relieve travelers' diarrhea, antibiotic-associated diarrhea, atopic eczema, and irritable bowel syndrome (IBS)9). Infants with AD or other allergic diseases show less frequent intestinal colonization with probiotics such as Lactobacillus or Bifidobacterium and more frequent colonization with Clostridium relative to non-allergic infants10). Recently, the administration of probiotic bacteria was reported to help maintain anti-inflammatory and tolerant immunity, which resulted in a lower prevalence of allergy in human subjects (Table 1). Potential mechanisms explaining the health-promoting actions of probiotic bacteria may include modulation of the intestinal immune system and displacement of potential pathogens via competitive exclusion or the production of antimicrobial agents. The efficacy of probiotics in the prevention of AD was primarily observed in infants who were administered probiotics during the perinatal period. Hattori et al.31) reported that children with AD and with a low degree of intestinal Bifidobacterium colonization experienced an amelioration of their allergic symptoms when lyophilized Bifidobacterium was administered orally. Systematic analysis in some clinical studies suggested that the intake of probiotics by mothers during pregnancy reduces the incidence of subsequent eczema5). Young children with immunoglobulin E (IgE)-mediated atopic eczema, in particular, showed more significant improvement with the administration of probiotics32). Other studies have also shown that the adult immune system is more difficult to modulate by the administration of probiotics. An analysis of systematic reviews suggests that there is little evidence to support a clinically useful benefit of using probiotics in patients with established eczema, although immunomodulation may also occur in adulthood5). No beneficial effect was found from B. lactis or L. paracasei supplementation in the treatment of eczema when given as an adjunct to basic topical treatment, and no effect on the progression of allergic disease from age 1 to 3 years was noted11). The preventive effect of probiotics is strain specific; when L. rhamnosus HN001 and B. animalis subsp. lactis HN019 were compared, only L. rhamnosus HN001 reduced (by 2 years) the cumulative prevalence of eczema in infants at risk of allergic disease33). In various studies, the tested probiotic mixtures contained different strains, and individual strains were not tested. Therefore, it is still premature to conclude that individual component strain can have a synergistic effect when combined into a mixture, as comparisons of the effect of a probiotic mixture with that of one or more of its component strains have not been performed, particularly for severe diseases. Early life administration of a cow's milk formula supplemented with B. longum BL999 and L. rhamnosus LPR showed no effect on the prevention of eczema or allergen sensitization in the first year of life in infants at risk for allergic disease29).
Occasionally, an identical strain shows contradictory results. In the case of L. rhamnosus GG, early reports suggested that L. rhamnosus GG (1×1010 colony-forming units [CFUs] of L. rhamnosus GG daily) administered to pregnant mothers and subsequently to infants after delivery reduced the incidence of AD by half relative to those treated with placebo34). However, a more recent study that employed a nearly identical study design showed that supplementation of L. rhamnosus GG (5×109 CFUs twice daily during pregnancy and early infancy) did not reduce the incidence and the severity of AD in affected children. Rather, probiotic supplementation was associated with an increased rate of recurrent episodes of wheezing bronchitis35). Moreover, oral administration of L. rhamnosus GG in a prospective, double-blind, randomized, placebo-controlled study had no clinical effect on AD or asthma-related events in young children (6 to 24 months old) with recurrent wheezing and a family history of atopy25).
Although a meta-analysis revealed the positive potential of probiotics, the mechanism of action or biomarkers related to their anti-AD effect were not clarified. The reduction of AD prevalence in infants with a family history of allergic diseases by the administration of a probiotic mixture (B. bifidum BGN4, B. lactis AD011, and L. acidophilus AD031) was associated with significant increases in the capacity of transforming growth factor beta (TGF-β) production by peripheral blood mononuclear cells36). When 62 mother and infant pairs were supplemented with probiotics during pregnancy and their breastfeeding period, the level of TGF-β2 was higher in the breast milk from mothers in the probiotics group than in that from mothers in the control group37). Compared with a placebo, the administration of L. sakei KCTC 10755BP to children aged 2 to 10 years with atopic eczema-dermatitis syndrome and a minimum SCORing of Atopic Dermatitis (SCORAD) score of 25 resulted in a decreased SCORAD total score associated with lower pretreatment-adjusted serum levels of chemokine (c-c motif) ligand CCL17 and CCL27, which are chemokines involved in the process of establishing inflammatory infiltration of cells27). Oral administration of combined L. rhamnosus and L. reuteri improved the extent of the eczema and decreased serum eosinophil cationic protein levels in children38); this effect was more pronounced in patients with a positive skin prick test response and elevated IgE levels. Supplementation of B. lactis Bb-12 or L. rhamnosus GG to infants with atopic eczema during the weaning period reduced the extent and the severity of atopic eczema, which was accompanied by the reduction of serum cluster of differentiation (CD)4 and urine eosinophilic protein X39). Taken together, the results from the 3 clinical studies described above suggest that probiotics improve the symptoms of inflammatory responses in allergic diseases beyond the intestinal milieu39). An increase in the traffic of circulating CD34+ hematopoietic precursor cells (HPCs) was suggested to be a factor in systemic allergic inflammation40). In 14 allergic patients who were 6 to 48 years old with clinical symptoms of asthma and/or conjunctivitis, rhinitis, urticaria, AD, food allergy, and IBS, the number of circulating CD34+ HPC was decreased when a mixture of L. acidophilus , L. delbrueckii , and Streptococcus thermophilus was administered for 30 days40). A study to assess whether the administration of probiotics affects the microbiota and its genotoxic activity in healthy subjects and patients with AD revealed that the administration of a probiotic mix containing L. paracasei Lpc-37, L. acidophilus 74-2, and B. animalis subsp. lactis DGCC 420 decreased the genotoxic potential of fecal water in AD patients. The fecal C. perfringens cluster I-II levels remained unaffected, suggesting either a change in their activity or that other bacterial species are responsible for the reduced genotoxic activity of fecal water12). In an allergic condition, the function of Tregs and their production of cytokines such as interleukin (IL)-10 and TGF-β are dysregulated compared to the normal condition, resulting in prolonged inflammatory responses against environmental allergens41). The intestinal epithelial cells secrete thymic stromal lymphopoietin, TGF-β, and retinoic acid, which induce the development of resident CD11b regulatory dendritic cells (DCs), which in turn induce the development of naive T cells into forkhead box family transcription factor Foxp3+ Tregs42). One plausible reason as to why the administration of probiotics can downregulate both Th2-related allergy and Th1-related inflammatory symptoms is related to the action of probiotics to improve regulatory immune activity, as evidenced by the results of animal experiments43). Actually, low-grade inflammation was suggested as a key factor, not only in the pathogenesis of AD but also in IBS. Consistent with this, the administration of B. bifidum BGN4-containing probiotics improved both AD and irritable syndrome in 2 separate clinical trials, as described below. In double-blind, randomized, placebo-controlled human trials, infants who were perinatally administered a combination of B. bifidum BGN4, B. lactis AD011, and L. acidophilus AD031 showed significantly lower prevalence and cumulative incidence of AD than a placebo group26). In a prospective, double-blind, randomized, placebo-controlled clinical study, IBS patients that received composite probiotics (B. bifidum BGN4, B. lactis AD011, L. acidophilus AD031, and L. casei IBS041) showed significant reductions in their IBS symptoms, including abdominal pain, after 8 weeks of treatment. This was observed particularly in the patients with mixed or diarrhea-predominant ailments44). However, an analysis of the function of the Tregs and the expression of Foxp3+ does not have close clinical relevance when judging the efficacy of probiotics in AD patients, due to the various conflicting results pertaining to the relationship between the function of the regulatory cells and the occurrence of allergic symptoms. The suppressive function of the Tregs was diminished in infants with egg allergies45). Paradoxically, CD4+CD25+ Tregs expressing Foxp3+ were increased in patients with AD compared to normal individuals46). Likewise, children with AD had significantly higher induced Foxp3+ expression following stimulation with both house dust mites and ovalbumin (OVA) allergens compared to those without AD, which was suggested to reflect secondary compensatory mechanisms47). In addition, the administration of L. acidophilus LAVRI-A1 did not have significant effects on CD4+CD25+CTLA4+ cell numbers or Foxp3+ expression in high-risk children47).
In addition to probiotics, prebiotics have also shown some efficacy in ameliorating AD. When the effects of L. salivarius and fructooligosaccharide (synbiotic) with fructooligosaccharide alone (prebiotic) were compared in children with moderate to severe AD, the synbiotic combination was superior to the prebiotic alone for treating moderate to severe childhood AD48).
Preschool children receiving synbiotics (L. acidophilus DDS-1, B. lactis UABLA-12 with fructooligosaccharide) showed a greater decrease in the mean SCORAD score and need for topical corticosteroids than children in the placebo group after 8 weeks. Interestingly, a flow cytometric analysis of lymphocyte subsets in the peripheral blood of patients in the probiotic group showed that the percentage of CD4 and the percentage and absolute count of CD25 decreased whereas the percentage and absolute count of CD8 increased24).
While most of the clinical studies used live forms of probiotic bacteria, Hoang et al.21) used the cell lysate of L. rhamnosus, reporting a substantial improvement in the quality of life, skin symptoms, and day and night-time irritation scores in children that received supplementation; however, this study was limited in its meaningfulness due to its open label, non-randomized clinical observation. The skin severity scores of AD decreased in the adult patients from baseline values at week 8 and week 12 when the subjects were given a diet containing heat-killed L. paracasei . However, the effect was largely limited because there was no significant difference between the Lactobacillus and placebo groups20). The administration of heat-killed Lactobacillus or administration onto established eczema may be a factor related to its weak effect observed in the study.
An effect of probiotics on the improvement of allergic diseases other than AD has also been reported. Daily supplementation with L. gasseri A5 for 8 weeks improved the clinical symptoms and immunoregulatory changes in school children suffering from asthma and AR23). The prevalence of "frequent wheezing" and "wheezing and/or noisy breathing apart from colds" was significantly lower in the synbiotic (B. breve M-16V and a galacto/fructooligosaccharide mixture) than in the placebo group, despite the fact that the total IgE levels did not differ between the groups19).
Recently, numerous publications have supported the effect of probiotics on the prevention and treatment of allergic diseases in animal studies. However, the suggested mechanisms related to their anti-allergic effects were variable. The action of probiotics to shift the immune system from the pathogenic Th2 response to a Th1/T regulatory response was demonstrated (Fig. 1). Oral treatment with the probiotic mixture VSL#3 was effective in redirecting allergen-specific Th2 polarized immune responses towards Th1/T regulatory responses. This treatment also offered protection against allergen-induced anaphylactic reactions in a murine model of food allergy49). The oral administration of L. rhamnosus GG in OVA-immunized rats induced OVA-specific hyporesponsiveness and reduced the OVA-induced proliferative response in mesenteric lymph nodes (MLNs) associated with CD4+CD25+Foxp3+ T cell expansion and increased IL-10 and TGF-β secretion51). Exposure to the commensals and saprophytes in the absence of true danger signals from invasive pathogens and/or injured host cells were reported to induce the actions of regulatory network41) such as Tregs and inducible Tregs (Th3, Tr1) as well as Foxp3+. Foxp3+ is crucial for both the differentiation of Tregs and the maintenance of their suppressive function52). The induction of bacterial strain-specific Foxp3+ Tregs was evident in mice treated with B. longum AH1206 but not in mice treated with L. salivarius AH102, suggesting that the induction of Foxp3+ Tregs was strain specific53). The regulatory network including, in addition to Tregs, DCs and the cytokines produced by these cells is essential in the development of tolerance. Kwon et al.43) showed that IRT5, a probiotic mixture, exerted potent immunomodulatory effects by upregulating or enhancing the generation of Tregs by tolerogenic DCs in MLN. Moreover, the migration of CD4+Foxp3+ Tregs to sites of inflammation effectively suppressed disease progression. The enhanced therapeutic efficacy was associated with an increase in anti-inflammatory cytokines (IL-10 and TGF-β) as well as a decrease in pro-inflammatory cytokines.
The effect of L. casei in inhibiting allergic inflammation by acting at the effector phase of adaptive immune responses instead of at the initiation phase was also reported54). The suppressive effects of L. gasseri OLL2809 on inflammatory responses was associated with the suppression of CD4+ T cell proliferation through a MyD88-dependent signaling pathway and by L. gasseri OLL2809 and its RNA55).
The oral treatment of neonatal pigs with L. lactis significantly reduced the subsequent frequency of allergy to ovomucoid and was associated with lower IgG(1)/IgG(2) and IgE/IgG(2) ratios, indicating a Th1 bias and a reduced Th2 immune response56). Kim et al.57) showed that B. bifidum BGN4 and L. casei appeared to be useful probiotic bacteria for the prevention of allergy, suggesting that these bacteria induce anti-allergenic processes through the induction of the Th1 response and the regulatory lymphocyte. Pochard et al.58) demonstrated that L. plantarum, L. lactis , L. casei , and L. rhamnosus GG suppressed IL-4 and IL-5 (Th2 cytokines) and increased interferon-gamma (IFN-γ) and IL-12 (Th1 cytokines) in a dose-dependent manner, suggesting a more balanced Th1/Th2 response in vitro with human polymorphonuclear cells.
The observation that probiotics enhanced the production of Th1 and regulatory cytokines in vitro but slightly decreased the ex vivo production of IL-10, tumor necrosis factor-alpha, and IL-6 suggests that the effect of probiotics with regard to the immunomodulatory potential differed depending on the in vitro or ex vivo treatment59). Moreover, changes in the production of various cytokines and the activation of immunoregulatory cells as observed in animal studies have not been revealed in human studies.
Various meta-analysis and systematic review studies have shown positive effects of probiotics with regard to the prevention of AD, particularly in infants who were administered probiotics during the perinatal period. However, further studies regarding the optimal dose, effective probiotic strains, the timing and duration of supplementation, the additive/synergistic effects between probiotics and prebiotics, and patient populations that would most benefit from the use of probiotics need to be more thoroughly investigated. While there is substantial evidence for the amelioration of AD by probiotics from in vitro experiments and animal studies, the results from human clinical trials are more complicated. Thus elucidating the effects and mechanisms of action of probiotics is difficult due to the variation of results derived from human studies. Therefore, the mechanism involved in the preventive effect of probiotics in humans and the long-term effects of probiotics on the developing immune system remain to be proven.
Acknowledgment
This study was supported by the Small and Medium Business Administration (No. S1072365) and the Next-Generation BioGreen 21 Program (No. PJ008005), from the Rural Development Administration of the Republic of Korea.
References
1. Saeki H, Furue M, Furukawa F, Hide M, Ohtsuki M, Katayama I, et al. Guidelines for management of atopic dermatitis. J Dermatol 2009;36:563–577.


2. Asher MI, Montefort S, Bjorksten B, Lai CK, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 2006;368:733–743.


3. Williams H, Stewart A, von Mutius E, Cookson W, Anderson HR. International Study of Asthma and Allergies in Childhood (ISAAC) Phase One and Three Study Groups. Is eczema really on the increase worldwide? J Allergy Clin Immunol 2008;121:947–954.e15.


4. Williams H, Robertson C, Stewart A, Ait-Khaled N, Anabwani G, Anderson R, et al. Worldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. J Allergy Clin Immunol 1999;103(1 Pt 1): 125–138.


5. Batchelor JM, Grindlay DJ, Williams HC. What's new in atopic eczema? An analysis of systematic reviews published in 2008 and 2009. Clin Exp Dermatol 2010;35:823–827.


7. Akdis CA, Blaser K, Akdis M. Apoptosis in tissue inflammation and allergic disease. Curr Opin Immunol 2004;16:717–723.


8. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria: report of a joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. World Health Organization, Food and Agriculture Organization of the United Nations [Internet]. c2012;2012 Jan 20. Geneva: WHO/FAO, Available from: http://www.who.int/foodsafety/publications/fs_management/en/probiotics.pdf.
9. Chapman CM, Gibson GR, Rowland I. Health benefits of probiotics: are mixtures more effective than single strains? Eur J Nutr 2011;50:1–17.


10. Bjorksten B, Sepp E, Julge K, Voor T, Mikelsaar M. Allergy development and the intestinal microflora during the first year of life. J Allergy Clin Immunol 2001;108:516–520.


11. Gore C, Custovic A, Tannock GW, Munro K, Kerry G, Johnson K, et al. Treatment and secondary prevention effects of the probiotics Lactobacillus paracasei or Bifidobacterium lactis on early infant eczema: randomized controlled trial with follow-up until age 3 years. Clin Exp Allergy 2012;42:112–122.


12. Roessler A, Forssten SD, Glei M, Ouwehand AC, Jahreis G. The effect of probiotics on faecal microbiota and genotoxic activity of faecal water in patients with atopic dermatitis: a randomized, placebo-controlled study. Clin Nutr 2012;31:22–29.


13. Larsen N, Vogensen FK, Gobel R, Michaelsen KF, Abu Al-Soud W, Sorensen SJ, et al. Predominant genera of fecal microbiota in children with atopic dermatitis are not altered by intake of probiotic bacteria Lactobacillus acidophilus NCFM and Bifidobacterium animalis subsp. lactis Bi-07. FEMS Microbiol Ecol 2011;75:482–496.


14. Morisset M, Aubert-Jacquin C, Soulaines P, Moneret-Vautrin DA, Dupont C. A non-hydrolyzed, fermented milk formula reduces digestive and respiratory events in infants at high risk of allergy. Eur J Clin Nutr 2011;65:175–183.


15. Torii S, Torii A, Itoh K, Urisu A, Terada A, Fujisawa T, et al. Effects of oral administration of Lactobacillus acidophilus L-92 on the symptoms and serum markers of atopic dermatitis in children. Int Arch Allergy Immunol 2011;154:236–245.


16. Boyle RJ, Ismail IH, Kivivuori S, Licciardi PV, Robins-Browne RM, Mah LJ, et al. Lactobacillus GG treatment during pregnancy for the prevention of eczema: a randomized controlled trial. Allergy 2011;66:509–516.


17. Kukkonen AK, Savilahti EM, Haahtela T, Savilahti E, Kuitunen M. Ovalbumin-specific immunoglobulins A and G levels at age 2 years are associated with the occurrence of atopic disorders. Clin Exp Allergy 2011;41:1414–1421.


18. Nermes M, Kantele JM, Atosuo TJ, Salminen S, Isolauri E. Interaction of orally administered Lactobacillus rhamnosus GG with skin and gut microbiota and humoral immunity in infants with atopic dermatitis. Clin Exp Allergy 2011;41:370–377.


19. van der Aa LB, van Aalderen WM, Heymans HS, Henk Sillevis Smitt J, Nauta AJ, Knippels LM, et al. Synbiotics prevent asthma-like symptoms in infants with atopic dermatitis. Allergy 2011;66:170–177.


20. Moroi M, Uchi S, Nakamura K, Sato S, Shimizu N, Fujii M, et al. Beneficial effect of a diet containing heat-killed Lactobacillus paracasei K71 on adult type atopic dermatitis. J Dermatol 2011;38:131–139.


21. Hoang BX, Shaw G, Pham P, Levine SA. Lactobacillus rhamnosus cell lysate in the management of resistant childhood atopic eczema. Inflamm Allergy Drug Targets 2010;9:192–196.


22. Dotterud CK, Storro O, Johnsen R, Oien T. Probiotics in pregnant women to prevent allergic disease: a randomized, double-blind trial. Br J Dermatol 2010;163:616–623.


23. Chen YS, Jan RL, Lin YL, Chen HH, Wang JY. Randomized placebo-controlled trial of Lactobacillus on asthmatic children with allergic rhinitis. Pediatr Pulmonol 2010;45:1111–1120.


24. Gerasimov SV, Vasjuta VV, Myhovych OO, Bondarchuk LI. Probiotic supplement reduces atopic dermatitis in preschool children: a randomized, double-blind, placebo-controlled, clinical trial. Am J Clin Dermatol 2010;11:351–361.


25. Rose MA, Stieglitz F, Koksal A, Schubert R, Schulze J, Zielen S. Efficacy of probiotic Lactobacillus GG on allergic sensitization and asthma in infants at risk. Clin Exp Allergy 2010;40:1398–1405.


26. Kim JY, Kwon JH, Ahn SH, Lee SI, Han YS, Choi YO, et al. Effect of probiotic mix (Bifidobacterium bifidum, Bifidobacterium lactis, Lactobacillus acidophilus) in the primary prevention of eczema: a double-blind, randomized, placebo-controlled trial. Pediatr Allergy Immunol 2010;21(2 Pt 2): e386–e393.


27. Woo SI, Kim JY, Lee YJ, Kim NS, Hahn YS. Effect of Lactobacillus sakei supplementation in children with atopic eczema-dermatitis syndrome. Ann Allergy Asthma Immunol 2010;104:343–348.


28. Niers L, Martin R, Rijkers G, Sengers F, Timmerman H, van Uden N, et al. The effects of selected probiotic strains on the development of eczema (the PandA study). Allergy 2009;64:1349–1358.


29. Soh SE, Aw M, Gerez I, Chong YS, Rauff M, Ng YP, et al. Probiotic supplementation in the first 6 months of life in at risk Asian infants: effects on eczema and atopic sensitization at the age of 1 year. Clin Exp Allergy 2009;39:571–578.


30. Kuitunen M, Kukkonen K, Juntunen-Backman K, Korpela R, Poussa T, Tuure T, et al. Probiotics prevent IgE-associated allergy until age 5 years in cesarean-delivered children but not in the total cohort. J Allergy Clin Immunol 2009;123:335–341.


31. Hattori K, Yamamoto A, Sasai M, Taniuchi S, Kojima T, Kobayashi Y, et al. Effects of administration of bifidobacteria on fecal microflora and clinical symptoms in infants with atopic dermatitis. Arerugi 2003;52:20–30.

32. Savilahti E, Kuitunen M, Vaarala O. Pre and probiotics in the prevention and treatment of food allergy. Curr Opin Allergy Clin Immunol 2008;8:243–248.


33. Wickens K, Black PN, Stanley TV, Mitchell E, Fitzharris P, Tannock GW, et al. A differential effect of 2 probiotics in the prevention of eczema and atopy: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol 2008;122:788–794.


34. Kalliomaki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet 2001;357:1076–1079.


35. Kopp MV, Hennemuth I, Heinzmann A, Urbanek R. Randomized, double-blind, placebo-controlled trial of probiotics for primary prevention: no clinical effects of Lactobacillus GG supplementation. Pediatrics 2008;121:e850–e856.


36. Kim JY, Choi YO, Kwon JH, Ahn KM, Park MS, Ji GE. Clinical effects of probiotics are associated with increased transforming growth factor-β responses in infants with high-risk allergy. J Korean Soc Appl Biol Chem 2011;54:944–948.

37. Rautava S, Kalliomaki M, Isolauri E. Probiotics during pregnancy and breast-feeding might confer immunomodulatory protection against atopic disease in the infant. J Allergy Clin Immunol 2002;109:119–121.


38. Rosenfeldt V, Benfeldt E, Nielsen SD, Michaelsen KF, Jeppesen DL, Valerius NH, et al. Effect of probiotic Lactobacillus strains in children with atopic dermatitis. J Allergy Clin Immunol 2003;111:389–395.


39. Isolauri E, Arvola T, Sutas Y, Moilanen E, Salminen S. Probiotics in the management of atopic eczema. Clin Exp Allergy 2000;30:1604–1610.

40. Mastrandrea F, Coradduzza G, Serio G, Minardi A, Manelli M, Ardito S, et al. Probiotics reduce the CD34+ hemopoietic precursor cell increased traffic in allergic subjects. Eur Ann Allergy Clin Immunol 2004;36:118–122.

41. von Hertzen LC, Savolainen J, Hannuksela M, Klaukka T, Lauerma A, Mäkela MJ, et al. Scientific rationale for the Finnish Allergy Programme 2008-2018: emphasis on prevention and endorsing tolerance. Allergy 2009;64:678–701.


42. Ng SC, Kamm MA, Stagg AJ, Knight SC. Intestinal dendritic cells: their role in bacterial recognition, lymphocyte homing, and intestinal inflammation. Inflamm Bowel Dis 2010;16:1787–1807.


43. Kwon HK, Lee CG, So JS, Chae CS, Hwang JS, Sahoo A, et al. Generation of regulatory dendritic cells and CD4+Foxp3+ T cells by probiotics administration suppresses immune disorders. Proc Natl Acad Sci U S A 2010;107:2159–2164.



44. Hong KS, Kang HW, Im JP, Ji GE, Kim SG, Jung HC, et al. Effect of probiotics on symptoms in Korean adults with irritable bowel syndrome. Gut Liver 2009;3:101–107.



45. Smith M, Tourigny MR, Noakes P, Thornton CA, Tulic MK, Prescott SL. Children with egg allergy have evidence of reduced neonatal CD4(+) CD25(+)CD127(lo/-) regulatory T cell function. J Allergy Clin Immunol 2008;121:1460–1466. 1466.e1–1466.e7.


46. Ou LS, Goleva E, Hall C, Leung DY. T regulatory cells in atopic dermatitis and subversion of their activity by superantigens. J Allergy Clin Immunol 2004;113:756–763.


47. Taylor AL, Hale J, Hales BJ, Dunstan JA, Thomas WR, Prescott SL. FOXP3 mRNA expression at 6 months of age is higher in infants who develop atopic dermatitis, but is not affected by giving probiotics from birth. Pediatr Allergy Immunol 2007;18:10–19.

48. Wu KG, Li TH, Peng HJ. Lactobacillus salivarius plus fructo-oligosaccharide is superior to fructo-oligosaccharide alone for treating children with moderate to severe atopic dermatitis: a double-blind, randomized, clinical trial of efficacy and safety. Br J Dermatol 2012;166:129–136.


49. Schiavi E, Barletta B, Butteroni C, Corinti S, Boirivant M, Di Felice G. Oral therapeutic administration of a probiotic mixture suppresses established Th2 responses and systemic anaphylaxis in a murine model of food allergy. Allergy 2011;66:499–508.


50. Gomez-Llorente C, Munoz S, Gil A. Role of Toll-like receptors in the development of immunotolerance mediated by probiotics. Proc Nutr Soc 2010;69:381–389.


51. Finamore A, Roselli M, Britti MS, Merendino N, Mengheri E. Lactobacillus rhamnosus GG and Bifidobacterium animalis MB5 induce intestinal but not systemic antigen-specific hyporesponsiveness in ovalbumin-immunized rats. J Nutr 2012;142:375–381.


53. Lyons A, O'Mahony D, O'Brien F, MacSharry J, Sheil B, Ceddia M, et al. Bacterial strain-specific induction of Foxp3+ T regulatory cells is protective in murine allergy models. Clin Exp Allergy 2010;40:811–819.


54. Schiffer C, Lalanne AI, Cassard L, Mancardi DA, Malbec O, Bruhns P, et al. A strain of Lactobacillus casei inhibits the effector phase of immune inflammation. J Immunol 2011;187:2646–2655.


55. Yoshida A, Yamada K, Yamazaki Y, Sashihara T, Ikegami S, Shimizu M, et al. Lactobacillus gasseri OLL2809 and its RNA suppress proliferation of CD4(+) T cells through a MyD88-dependent signalling pathway. Immunology 2011;133:442–451.



56. Rupa P, Schmied J, Wilkie BN. Prophylaxis of experimentally induced ovomucoid allergy in neonatal pigs using Lactococcus lactis. Vet Immunol Immunopathol 2011;140:23–29.


57. Kim H, Kwack K, Kim DY, Ji GE. Oral probiotic bacterial administration suppressed allergic responses in an ovalbumin-induced allergy mouse model. FEMS Immunol Med Microbiol 2005;45:259–267.


Fig. 1
Schematic view of the potential mechanism of action by which commensal bacteria and pathogenic bacteria interact with Toll-like receptors (TLRs) and elicit different immune responses. (A) Commensal and probiotic bacteria interact with intestinal epithelial-cell barrier and dendritic cells (DCs) resident in the intestine. Some cytokines, including interleukin (IL)-10, transforming growth factor beta (TGF-β) and thymic stromal lymphopoietin (TSLP), are expressed in intestinal epithelial cells, as a result of their interactions. Stimulation of cell TLR mediated by bacteria leads to up-regulation of TGF-β and IL-10, which in turn may limit the responsiveness of intestinal DCs resulting in the expansion and/or survival of T-cells with regulatory capacities, and limiting the ability of driving Th1, Th2 and Th17-cell responses. (B) Pathogenic bacteria have virulence factors that interact with intestinal epithelial-cell barrier and DCs resident in the intestine. Invasion of epithelium and direct interaction with DCs lead to activation of TLR and enhanced production of pro-inflammatory cytokines including interferon-gamma (IFN-γ) and IL-12, which are capable of driving Th1, Th2 and Th17 response. RA, retinoic acid; sIgA, secreted Ig A; Th, T helper cell; Treg, T regulatory cell (Reprinted from Gomez-Llorente C, Munoz S, Gil A. Proc Nutr Soc 2010; 69:381-9, with permission of Cambridge University Press)50).
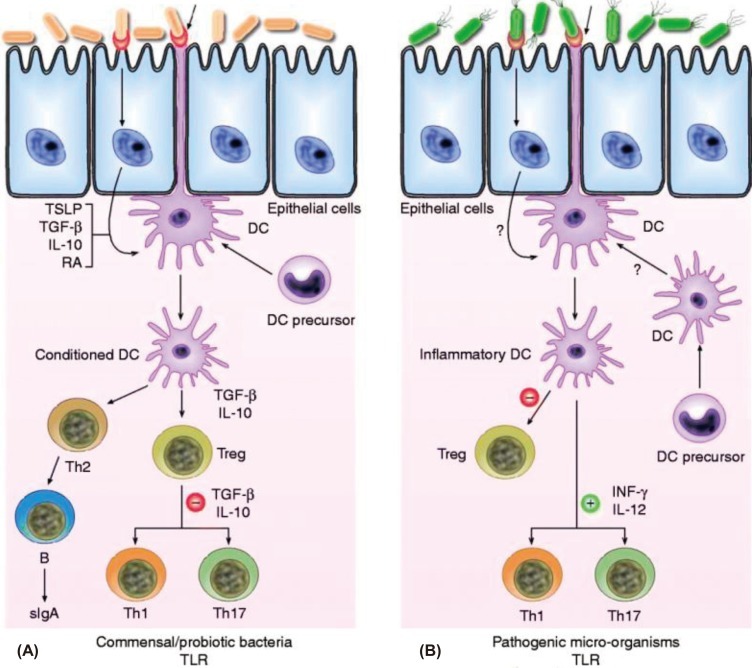
Table 1
The Effects of Probiotics on Allergic Diseases in Human Clinical Trials (since 2009)
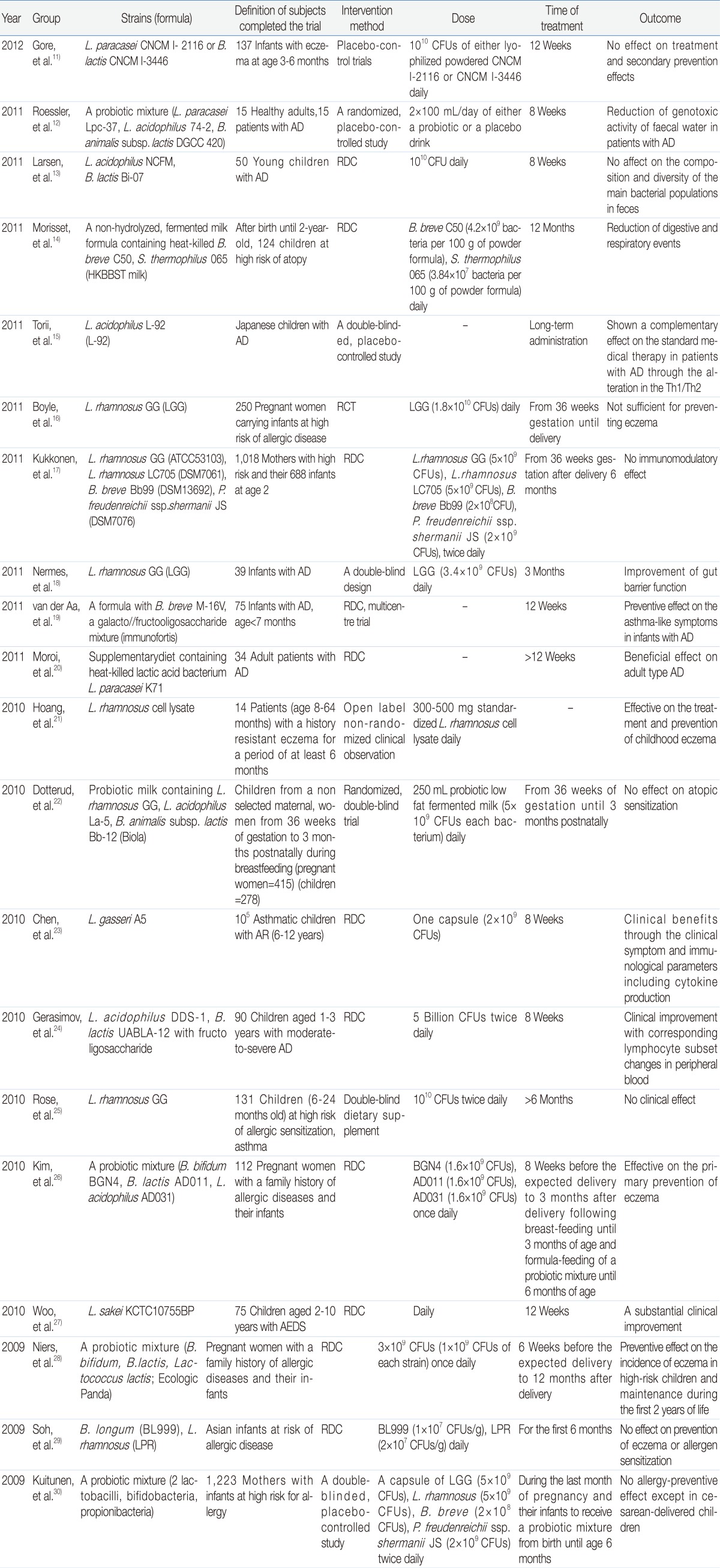
L., Lactobacillus ; B., Bifidobacterium; S., Streptococcus ; P., Propionibacterium; CFU, colony-forming unit; AD, atopic dermatitis; AR, allergic rhinitis; AEDS, atopic eczema-dermatitis syndrome; RDC, a randomized, double-blind, placebo-controlled study; RCT, a randomized, placebo-controlled trials.



 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


