Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is the only curative option for many diseases, such as hematologic malignancies, immune deficiency syndrome, and bone marrow (BM) failure. A human leukocyte antigen (HLA)-matched sibling donor is considered the best hematopoietic stem cell (HSC) donor; however, only approximately 30% of the patients can receive transplantation from an HLA-matched sibling donor. In the absence of such a donor, the search for an unrelated volunteer adult donor is currently performed and more than 14 million potential unrelated adult donors are registered with the unrelated-donor registries worldwide. However, the HLA-matched unrelated donors are found for only approximately 50% of the patients. Additionally, the search and procurement process for adult donors can take weeks or months; consequently, cord blood (CB) is becoming an increasingly attractive alternative to HLA-matched peripheral blood progenitor cells (PBPCs) or BM.
History of cord blood transplantation (CBT)
Since the CB was found to have sufficient progenitor cells for clinical hematopoietic reconstitution, researches showed that CB could be an alternative HSC source1). Thereafter, studies about collection, transportation, optimal cryopreservation of CB, and the viability of CB after thawing have been conducted. In 1988, the first CBT was performed in a patient with Fanconi anemia (FA)2). Furthermore, in 1991, CB was successfully transplanted from an HLA-matched sibling to a child with acute lymphoblastic leukemia, and in 1993, CB was transplanted from an unrelated donor for the first time3). CB, which was previously discarded after birth, has emerged as a valuable stem cell source and has led to remarkable progress in the field of allogeneic HSCT. The progress in the field of CBT aroused huge interest in the establishment and development of CB banks worldwide. These banks play an important role in the CBT process. In 1998, the Netcord group was created to establish good practices in CB storage, to facilitate donor search, to improve the quality of grafts, and to establish procedures for bank accreditation. At present, it is estimated that more than 400,000 CB units are available for transplantation in more than 100 CB banks in many countries.
Characteristics of CB
Although CB contains one log fewer nucleated cells than BM or mobilized PBPC, it has a higher primitive HSC content and higher proliferative potential than either BM or mobilized PBPC. Therefore, CB may serve as a reliable source for HSCT despite the limited cell number.
The incidence of graft-versus-host disease (GVHD) is less than expected given the degree of HLA disparity in CBT. The exact reasons for the relatively low incidence of GVHD are unknown, but may result from the functional immaturity of infused lymphocytes, including decreased cytotoxicity, an altered cytokine profile, decreased HLA expression and increased regulatory T-cells4). Despite a low incidence of GVHD in CBT, graft-versus-leukemia effect is preserved, which may be associated with natural killer cells in CB5).
CB offers many advantages such as easy procurement with no risk for the donor, reduced risk of transmitting infections, and rapid availability of cryopreserved cell. CBT also permits transplantation in the absence of full HLA compatibility between donor and recipient. The disadvantages of CBT are the higher rate of graft failure, delayed hematopoietic recovery, the risk of genetic disorder transmission, and the inability to further obtain stem cells or donor lymphocyte infusion6).
Clinical outcomes of CBT
1. CBT in children
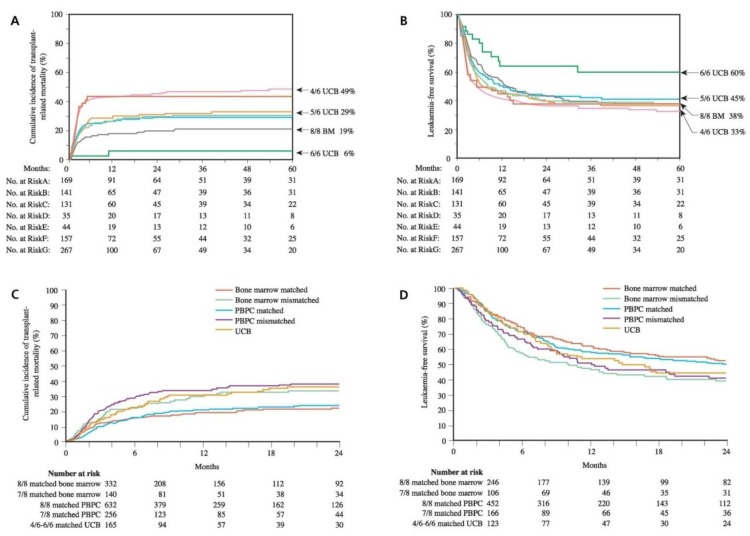
The International Bone Marrow Transplant Registry and Eurocord Transplant Group published the results of CBT from related donors, for which the 2-year survival rate was found to be 60%. In a study for children including 113 recipients of a related CBT and 2,052 recipients of related BM, CBT was found to be associated with delayed hematopoietic recovery and reduced acute and chronic GVHD. The 3-year survival rate was 64% in the recipients of CB and 66% in the recipients of BM, respectively. The relapse rate was same despite lower risk of GVHD in CBT than in bone marrow transplantation7). Overall, the data suggest that CB is as effective as BM as a source of HSC for children who receive transplants from HLA-identical siblings. This study was the basis for advocating the use of mismatched CBT and triggered the development of unrelated CB banks. In the unrelated donor transplant setting, CBT provides several advantages such as short time to transplant, rapid availability, tolerance to 1-2 HLA-mismatch, and a lower risk of GVHD. Cell dose and HLA compatibility are the major factors influencing the outcome after CBT. In a recent study, the outcomes of 503 children with acute leukemia and transplanted with CB were compared with outcomes of 282 BM recipients. In comparison with allele-matched BM transplants (HLA-A, B, C, DRB1; 8/8), the transplantation-related mortality (TRM) rates were higher after transplantation with 1 or 2-antigen HLA-mismatched CB and lower after transplantation with HLA-matched CB (Fig. 1A). The 5-year leukemia-free survival rate was similar between transplants of 1 or 2-antigen HLA-mismatched CB and transplants of allele-matched BM. Furthermore, the 5-year leukemia-free survival was higher after transplants of HLA-matched CB8). These data suggest that better HLA matching and higher cell doses significantly improve the outcomes (Fig. 1B). Therefore, greater investment in large-scale banking is required to increase HLA diversity and to search optimal CB unit.
2. CBT in adults
Cell dose is well recognized as a factor that influences outcomes following CBT. In particular, a low cell dose is a problem in adults, for whom it is difficult to find a single unit with an adequate cell dose for proceeding to transplantation. Despite cell dose limitation in adult CBT, a reduced incidence of GVHD in CBT may result in the reduced TRM in the recipients of CBT.
According to the study conducted by the Japan Cord Blood Bank Network, the results showed similar relapse rates, TRM, and overall survival rates between the recipients of CB and BM9). In a recent international retrospective analysis in adults with acute leukemia, 94% of the patients were transplanted with 1 or 2 antigen-mismatched CB and TRM was higher than in the recipients of 8/8 allele-matched PBPC or BM (Fig. 1C). However, the incidences of GVHD were lower in CB recipients than in the recipients of allele-matched PBPC or BM. As a result, the leukemia-free survival after CBT was comparable to that observed after allele-matched PBPC or BM transplantation (Fig. 1D). These data support the use of CB for adults when an HLA-matched unrelated adult donor is not available and when transplantation is urgent necessity10).
Clinical experiences of CBT in Korea
In 1996, the first CBT was attempted in a child with severe aplastic anemia, resulting in graft failure. A child with acute lymphoblastic leukemia achieved successful engraftment after CBT in 1998, but outcome was unsuccessful. Subsequently, a child with acute biphenotypic leukemia was successfully transplanted with CB and remained leukemia free11). However, CBT was not a realistic alternative to BM or PBPC transplantation in Korea until January 2003, when National Health Insurance started covering the cost of transplantation for children under the age of 16 years. Since then, the number of pediatric cases of CBT in Korea has increased dramatically. While 51 cases of CBT were performed for 7 years from 1996 to 2002, 49 cases were performed for one year of 2003. Many pediatric patients lacking a suitable donor can be transplanted and saved using CBT. In March 2004, the first successful double-unit CBT was accomplished in Korea for a 14-year-old girl with acute myeloid leukemia12). In January 2009, the Korean National Health Insurance policy began to cover all CB recipients, including adults without an appropriate familial donor. From 2001 to 2011, 352 cases of CBT have been performed through the KoreaCORD, of which 157 patients underwent double-unit CBT. A recent study reported the outcomes of 236 patients who underwent CBT, performed from 1996 to 2003 in 13 transplantation centers in Korea. The results showed that acute leukemia was the most frequent primary disease. The median times to neutrophil and platelet recovery were 18 and 45 days, respectively. Grade 2-4 acute GVHD and chronic GVHD developed in 41.1% and 36.1% of the cases, respectively. The 5-year overall survival and event-free survival rates were 47.5% and 36.9%, respectively. Cytomegalovirus (CMV) disease developed in 45 patients (21%) and was an adverse factor for survival. These data suggest that careful management for CMV is important in Korean children, whose CMV seroprevalence exceeds 90%. Because an advanced disease status was found to be the most important risk factor for poor survival in the study, timely CBT is recommended when an appropriate BM or PBPC donor is not available13).
Future directions
1. Double-unit CBT
Attempts to increase CB graft cell dose have led to phase I clinical trials testing the safety of combined transplantation of 2 partially HLA-matched CB units. Barker et al.14) studied 23 patients with high-risk hematologic malignancy who received 2 CB units after myeloablative conditioning. The engraftment rate was 91%. At day 60 after transplantation, engraftment was derived from a single donor in 16 of 18 patients, with 1 unit predominating in all patients by day 100. Neither nucleated or CD34+ cell doses nor HLA-matching predicted which unit would predominate. The incidence of grades III to IV acute GVHD was 13%. These investigators concluded that transplantation of 2 partially HLA-mismatched CB units is safe and improves engraftment without increasing GVHD. It is speculated that the non-sustained unit may facilitate engraftment of the predominating unit by immunologic mechanisms despite the low cell dose in each unit14).
Interestingly, the sustained hematopoiesis was derived from a single donor, which predominated as early as 3 weeks after transplantation. Although mechanisms that determine the fate of each donor are not yet known, Korean investigators have first reported that the number of post-thaw colony forming units of granulocyte-macrophage progenitors is the only significant factor predicting engraftment of the predominating unit15). Double-unit CBT may overcome the cell-dose barrier that limits the use of CB in many adults. In Korea, as of May 2011, 157 cases of double-unit CBT have been performed.
2. Ex vivo expansion of CB
With the development of various expansion methods, the number of nucleated cells in CB could be expanded by as much as 400-fold; however, ex vivo expanded CB failed to show durable engraftment16). In a recent clinical trial, ex vivo expanded and unmanipulated CB fractions are combined for transplantation. Clinical data suggested that CB that has been subject to ex vivo expansion dose provide more rapid initial hematopoietic reconstitution, while unmanipulated CB is the source of the long-term, sustainable hematopoiesis17). Ex vivo expansion technologies are under investigation to inhibit the differentiation of progenitor cells and to facilitate engraftment.
3. Co-transplantation of CB and mesenchymal stem cells (MSCs)
MSCs are known to have the properties of tissue regeneration and immnosuppression18). MSCs have been suggested to support the microenvironment of BM, which is injured by conditioning regimen19). In addition, immunosuppressive potential of MSCs may provide protection from immune-mediated injury to HSC in CB20). Therefore, co-transplantation of CB and MSCs is under investigation in an attempt to overcome the engraftment delay associated with CBT21). MSCs can be isolated from BM, CB, Wharton jelly, and adipose tissues. Clinical trials have been reported using MSCs from haploidentical donor. In addition, promising results, which co-transplantation of third-party CB-derived MSCs may enhance the engraftment of CB, are first reported by Korean investigators22). However, randomized trials are required to confirm the effects of MSCs on enhancing the engraftment of HSC.
4. CBT after reduced-intensity conditioning (RIC)
The development of RIC regimens allowed the treatment of older patients and those with associated comorbid conditions. The results from RIC CBT series show a shortened time to engraftment with TRM rates that generally do not exceed those seen with myeloablative CBT23). Although longer follow-up is needed, there does not seem to be a convincing trend toward an increase in the rate of relapse after RIC CBT. Comparisons between RIC and myeloablative CBT are under study with respect to survival, optimal cell dose, incidence of GVHD, and rate of infection.
5. Progress in CB banking
The banking process should be standardized for HLA testing, virus detection, and counting of CD34+ cells. A larger inventory of high quality CB units will allow the discovery of larger and better matched units.
6. Designer baby
In 1999, using preimplantation genetic diagnosis and in vitro fertilization techniques, embryos HLA-identical to an FA patient, but unaffected by FA, were selected for intrauterine transfer. The transfer of a single preselected embryo resulted in a full-term pregnancy. The patient underwent transplantation with her newborn sibling donor's HLA-identical CB. Currently, the patient is well and shows normal hematopoiesis24). Preselection of embryos not affected by a specific disease are technically feasible; however, ethical and legal issues should be discussed before wider application.
Conclusion
Unrelated CB has emerged as an alternative allogeneic stem cell source. CB is readily available and provides suitable HLA-matched donors for patients who require allogeneic transplantation. CBT provides timely transplantation for patients with an unstable disease and reduces risk of GVHD without increased risk of relapse. In the clinical reports of CBT, the survival rates were comparable between transplants from CB and unrelated PBPC or BM in children with acute leukemia. The low incidence of chronic GVHD in CBT may benefit the quality of life of long-term survivors. Despite the limited cell dose in adult CBT, it is expected that reduced GVHD in CBT may reduce the TRM. Patients who do not have a matched sibling donor should simultaneously search for unrelated adult donors and CB. CBT is recommended, when the transplantation is urgent and a CB unit of nucleated cells of more than 2.0×107/kg is available. With the rapid development in the field of CBT including biology of CB, clinical trials, and banking process of CB, future research will amplify the broad applications of CB and help greater numbers of future patients.



 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


