Introduction
Congenital coronary arteriovenous fistulas (CCAFs) are rare coronary artery abnormalities in which blood is shunted into a cardiac chamber or great vessel. The clinical features, which depend on the size and location of these fistulas, range from a continuous murmur to congestive heart failure. Although small fistulas may be closed spontaneously, larger or symptomatic fistulas may be considered for either surgical or transcatheter correction to prevent complications and progressive nature1). Recently, interventional occlusion of CCAFs has emerged as a well-accepted alternative to surgery using various devices including detachable balloons, different types of coils, the Amplantzer duct occluder and the Amplatzer vascular plug (AVP)2,3).
If the fistula itself is large and tortuous, it is generally recommended to occlude both side of the fistula with devices4). In approaches of transcatheter occlusion, the transarterial approach is an easier access than transveous. However, it may damage normal coronary artery and the mitral valve, especially in children. In contrast, the transvenous approach would enable cannulation of a relatively large catheter or sheath without causing potential damage to the femoral vessels or normal coronary arteries, and would enable release of the device, minimizing the blind pouch5).
We report the success of transvenous proximal embolization of a CCAF using single AVP in a 3-year-old child with cardiomegaly.
Case report
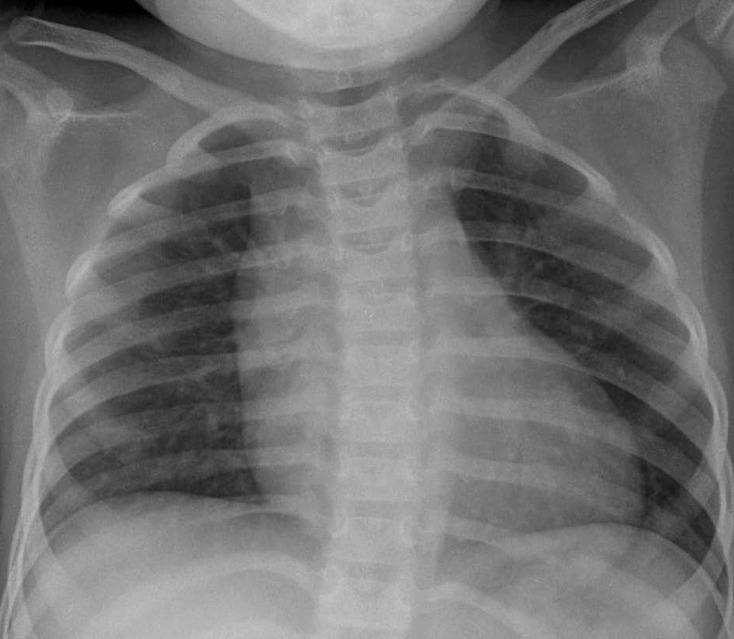
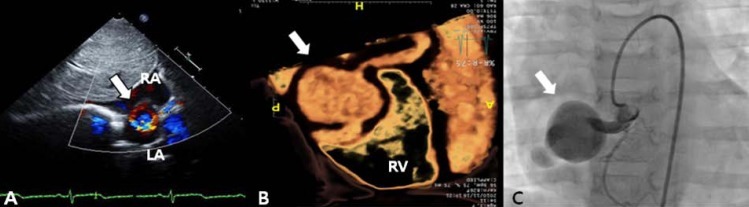
A 3-year-old girl, weighing 17 kg, was referred to Chonnam National University Hospital for evaluation of cardiomegaly. At 2-month of her age, the patient was evaluated for heart murmur and was diagnosed with CCAF. Because she did not show any symptoms of heart failure, medication was not given and serial echocardiography performed. On physical examination, grade 2/6 continuous murmur at the left lower sternal border was heard and she had no previous symptoms of tachypnea, poor feeding, cold sweat, cyanosis, etc. Chest radiography revealed cardiomegaly with normal pulmonary vascular markings (Fig. 1). Resting electrocardiogram (ECG) showed mild right ventricular hypertrophy and artery pressures were 90 mmHg systolic and 50 mmHg diastolic. Two-dimension and color Doppler ECG showed a coronary artery fistula arising from a dilated right coronary artery (RCA) and draining into the right atrium through the aneurysmal sac in the right atrium (Fig. 2A). Computed tomography showed an approximately 21×16 mm aneurysmal dilatation in a dilated sinoatrial nodal branch of the RCA, and a fistula was located between the distal portion of the aneurysmal sac and right atrium (Fig. 2B).
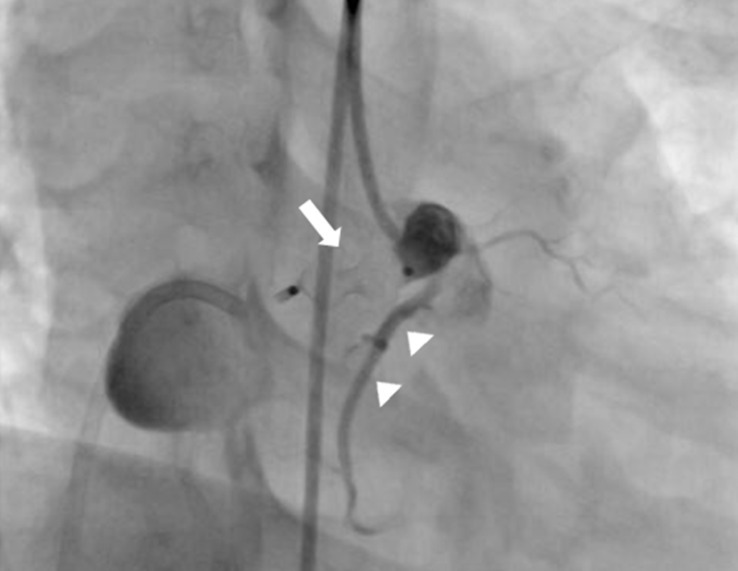
After parental informed consent, cardiac catheterization was performed. The right femoral vein and artery were cannulated and the patient was heparinized intravenously (50 IU/kg). A RCA angiogram revealed a dilated proximal RCA and 4 mm-sized coronary fistula originating from the proximal portion of the RCA emptying into the right atrium with an aneurysm having a maximal diameter of 23 mm; the sizes of the right marginal branch and RAC (originating distal to the fistula) were normal (Fig. 2C). After an unsuccessful attempt to guide the wire loop through the extensively tortuous fistula, we decided to change the approach to a transvenous approach. A Progreat 0.019 inch microcatheter (Terumo, Tokyo, Japan) was introduced into the right atrium through the right femoral vein, passing the aneurysm sac, coronary fistula, RCA and ascending aorta. A 0.014 inch runthrough wire (Terumo) was inserted along with the Progreat wire and was replaced with a 4F multipurpose catheter. It was then placed with its tip at the proximal coronary fistula. An 8-mm AVP, which is approximately twice as large as the diameter of the proximal fistula, was inserted and placed immediately proximal to the coronary fistula (at its origin from the normal RCA) in order to minimize the "blind pouch."
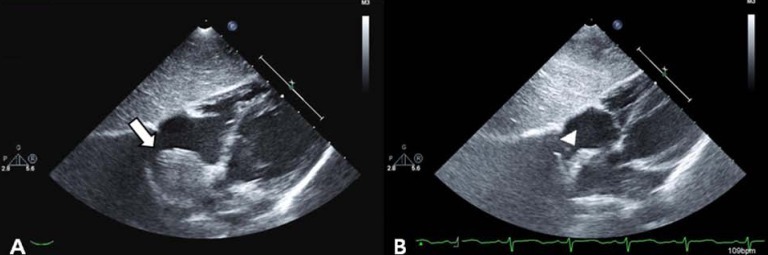
After confirmation of the correctly positioned AVP by a RCA angiogram without evidence of ischemic changes in an ECG, the device was released. In repeated RCA angiography 10 minutes after AVP insertion, no shunt lesion was noted (Fig. 3). A repeat echocardiography 3 days after intervention showed no shunt, although the fistula and thrombus were formed in the distal aneurysmal portion of the fistula (Fig. 4A). We did not attempt to occlude the distal fistula with another device anymore because distal portion was already naturally closed by the thrombus. The follow-up chest posteroanterior view showed decreased heart size after the procedure.
The patient was prescribed aspirin 3 mg/kg daily for 6 months to prevent thromboembolic events. Follow-up echocardiography at 1, 3, 6 months and 1 year after the procedure showed a decreasing size of aneurysm of fistula with thrombus formation (Fig. 4B). The patient remained completely asymptomatic including no thromboemboic event during the follow-up period of 1 year and 4 months.
Discussion
CCAFs are uncommon diseases and most are asymptomatic in infancy. However, their progressive nature and potential complications are sometimes fatal to patients1,6). Therefore, occlusion of symptomatic CCAF and asymptomatic CCAF with high flow shunting has been generally recommended7). Transcatheter closure is preferred over open surgery because it is less invasive and requires less recovery time. Reports have described catheter-based occlusion using a variety of techniques and devices4). Coils are used for small size of fistula, however, they are not eligible when CCAF is large and has complex anatomy, and there are also the possibility of multiple coil implantations for complete occlusion, which can increase the risk of coil embolization8). An Amplantzer duct occluder is used for larger CCAF, which is relatively easy to control and there is no need to implant multiple devices. However, it requires a relatively larger delivery catheter that can be difficult when confronted by tortuous fistulas9). Recently, AVP has been considered for larger and more tortuous fistulas. It is easy to deliver and has a wide range of device sizes. An operator could perform an angiography by the side arm of a Tuohy-Borst connection. This enables more precise positioning. Also its' retrieval decreases the risk of obstruction and migration10).
Transarterial or transvenous approaches are used to occlude CCAFs. If the fistulous orifice is easy to access, the transvenous approach is preferred. This would enable the cannulation of a relatively large catheter or sheath deeply without causing potential damage to the femoral artery or normal coronary artery, and would facilitate release of the device minimizing the blind pouch. However, it is almost impossible to enter a CCAF when the fistula is extensively tortuous. When the catheter cannot be delivered directly to the fistulous orifice, the arteriovenous loop formation is warranted11). The use of an arteriovenous wire loop delivers the devices through very small catheters and sheaths, and avoids potential damage to the femoral artery. However, it has a risk of damage to the arteriovenous valve5). In our case, our initial attempt to make an arteriovenous wire loop or transarterial approach was unsuccessful, which forced us to use the transvenous approach. Consequently, the blind pouch was minimized by the alternative approach.
If the fistula itself is large and tortuous, it is generally recommended that the fistula be closed from both sides with devices4). On report described a case with fistula occlusion by proximal and distal devices. The authors first used three Gianturco coils placed in the distal bulbous portion of the fistula, followed by an AVP placed in the most proximal part of the fistula to ensure the obliteration of the blind pouch12). Another study reported a failed attempt to close a large fistula by a distal implant of AVP. These authors implanted another AVP proximal segment of the fistula9). However, these procedures by transarterial approach have a potential, ambivalent risk of making a "blind pouch" with minimal invasion of coronary artery or invasion of normal coronary artery with minimal "blind pouch." In our case, the fistula was a relatively large (4 mm), long and tortuous aneurysmal sac (23 mm) arising close to the right atrial end. Initially, we decided to deliver AVP to the proximal part of the fistula and then placed another AVP distal to the distal aneurysmal sac. After the former AVP was deployed in the proximal fistula, RCA angiography documented complete fistula occlusion at the AVP level without significant residual shunt. Thus, there was no necessity to implant another AVP. Consequently, the thrombus formation occurred in the distal aneurysmal sac after proximal fistula occlusion by a single AVP.
We propose that the spontaneous thrombus formation in distal aneurismal sac likely resulted from the placement of the AVP in such a way that it reduced the velocity of blood flow and thrombogenic condition due to vascular irritation when the guide wire passed the aneurysmal sac. Although there was a risk of thrombus embolization after closure, that does not appear to have occurred, and the aneurysmal sac and fistula decreased in size with time.
We report the success of transvenous proximal closure of a CCAF using AVP in a 3-year-old patient with cardiomegaly. Complete occlusion was achieved by a single AVP and thrombus formation of distal aneurysmal portion of the fistula. To our best knowledge, this is the youngest reported case using AVP with transvenous approach in South Korea. We suggest that this strategy of closing the proximal end with dilated fistula using single AVP by the transvenous approach can be a good option in treating CCAFs in young child.



 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


