Article Contents
| Korean J Pediatr > Volume 55(8); 2012 |
Abstract
Symptomatic pulmonary arterial hypertension (PAH) in patients with isolated atrial septal defect (ASD) is rare during infancy. We report a case of isolated ASD with severe PAH in an infant who developed airway obstruction as cardiomegaly progressed. The patient presented with recurrent severe respiratory insufficiency and failure to thrive before the repair of the ASD. Echocardiography confirmed volume overload on the right side of heart and severe PAH (tricuspid regurgitation [TR] with a peak pressure gradient of 55 to 60 mmHg). The chest radiographs demonstrated severe collapse of both lung fields, and a computed tomography scan showed narrowing of the main bronchus because of an intrinsic cause, as well as a dilated pulmonary artery compressing the main bronchus on the left and the intermediate bronchus on the right. ASD patch closure was performed when the infant was 8 months old. After the repair of the ASD, echocardiography showed improvement of PAH (TR with a peak pressure gradient of 22 to 26 mmHg), and the patient has not developed recurrent respiratory infections while showing successful catch-up growth. In infants with symptomatic isolated ASD, especially in those with respiratory insufficiency associated with severe PAH, extrinsic airway compression should be considered. Correcting any congenital heart diseases in these patients may improve their symptoms.
Atrial septal defect (ASD) is a common congenital cardiac lesion with an incidence of 6 to 10%1). Natural history of isolated secundum ASD in infancy and early childhood is generally benign, and presentation of symptoms or pulmonary arterial hypertension (PAH) is unusual at this age. Elective closure of isolated secundum ASD is performed in the fourth or fifth year of life in case of right-sided volume overload, preferably through interventional device closure2). However, some patients require closure of ASD much earlier because of severe problems, such as failure to thrive, recurrent respiratory infections, or respiratory insufficiency. Sometimes left-to-right shunt can cause airway compression due to a dilated pulmonary artery. We experienced an infantile case in which a patient with an isolated secundum ASD had persistent PAH and collapse of both lungs due to extrinsic compression of both main bronchi by a dilated pulmonary artery and intra-pulmonary bronchial obstruction.
A 1-month-old corrected age, male infant weighing 3.4 kg was transferred to our institute because of respiratory insufficiency. He was prematurely born by Caesarean section with a birth weight of 1,070 g at 32+1 weeks of gestation, and was small for gestational age. The patient was admitted for 50 days including neonatal period and treated with O2 for the first 4 days after birth due to pneumonia. There was no evidence of hyaline membrane disease or bronchopulmonary dysplasia on chest radiographs and computed tomography (CT) also. On the physical examination, bronchial breathing sounds were increased with rhonci. Regular heart beats were heard with grade 2 systolic murmur on left upper sternal border. He was diagnosed as secundum ASD in the neonatal period. Echocardiography confirmed secundum ASD (diameter 7×12 mm), right-sided volume overload, tricuspid regurgitation (TR) with a peak pressure gradient of 55 to 60 mmHg, dilated main pulmonary artery (diameter 19 mm) and both pulmonary artery (diameter 11 to 12 mm). When the systolic pulmonary artery pressure was estimated from the peak flow velocity in the TR jet by using the modified Bernoulli equation, his pulmonary artery pressure was 58 to 65 mmHg.
At first admission, he was intubated and ventilator care was needed because of progressive respiratory distress. The chest radiographs demonstrated severe collapse of both lung fields at the time (Fig. 1). CT was performed to rule-out other diseases associated with PAH at the corrected age of 1 month. A CT scan showed a collapse in the dependent portion of the right lung and a total collapse in the lower lobe of the left lung. Narrowing of main bronchus and right intermediate bronchus were evident and an intrinsic cause like bronchomalacia was suspected (Fig. 2). Viral culture studies (respiratory syncytial virus, adenovirus, influenza A virus) were done; results were all negative. After conservative management, he was weaned off the ventilator within 2 weeks. The infant was prescribed with heart failure medications (digoxin 10 mcg/kg/day divided by 2, furosemide 1 mg/kg/day divided by 2, spironolactone 1 mg/kg/day divided by 2), and extensive physiotherapy of the lung was performed before he was discharged on the 42nd hospital day.
He was hospitalized 3 more times up until the corrected age of 4 months due to recurrent respiratory infection and respiratory distress. We expected catch-up growth and improvement of narrowed airway suspected due to bronchomalasia with patient growth. However, on the 4th hospital admission, he required mechanical ventilation for 40 days due to respiratory insufficiency. Follow-up echocardiograph showed sustained PAH with a TR pressure gradient of 60 mmHg. After he was weaned off mechanical ventilation, a follow-up CT scan was performed at the corrected age of 8 months, which revealed dilated pulmonary artery compressing the left main bronchus and right intermediate bronchus, but showed improvement of consolidation in both lungs and mild improvement of stenosis of the right main bronchus (Fig. 3). Up until the corrected age of 8 months the patient was underweight (5.6 kg) and less than the 3rd percentile of his age.
To correct these problems, ASD patch closure was performed at this stage. We recognized that he did not suffer from bronchomalacia because his respiratory distress improved after repair of ASD, and the intrinsic cause of airway compression suspected on initial CT scan was due to intra-pulmonary compression resulting from enlarged bronchial vessels and lymphatics. Postoperative echocardiogram showed improvement of TR with decreased peak pressure gradient (22 to 26 mmHg), immediately after the operation. Postoperative recovery was uneventful and he was extubated on the 3rd post-operative day. He was discharged from the hospital on the 22th postoperative day. The patient is doing well 29 months following surgery and is without any medication. Both lungs appear to be normally expanded, as demonstrated by chest X-ray taken 13 months after the operation (Fig. 4). Postoperative CT was not performed to confirm improvement of bronchial compression, because he did not suffer from any recurrent respiratory infections. At present (3 years and 2 months), he shows catch-up growth with 12 kg of body weight, which is between 3rd and 10th percentile of his age.
Our case reports a rare instance where an infant with isolated ASD presented with severe PAH, and showed symptoms of airway compression. Lammers et al.3) reported that most patients with symptomatic secundum ASD requiring early surgery in the first year of life had ASD as part of syndromatic diseases or malformation complexes, or with trisomy 21 which may be the cause of PAH. In another report, pulmonary vascular obstructive disease was considered to be the primary abnormality in these symptomatic infant with ASD4). Andrews et al.5) pointed out the need to search carefully for other underlying cardiac or pulmonary abnormalities and recommended closure of the ASD, if other causes for PAH are excluded.
Tracheo-bronchial compression is known to be a cause of symptomatic ASD, especially in those who show respiratory insufficiency. Extrinsic airway compression is usually due to a vascular sling, whereas other causes are less common. However, as a result of close proximity of the pulmonary arteries to the bronchi, large left-to-right shunts can compress the airways6). Airway compression in the setting of congenital heart disease can result in frequent upper respiratory infection, wheezing, pneumonia, atelectasis and hyperinflation. Wheezing can be the result of intra-luminal bronchial obstruction, interstitial edema, bronchial narrowing and airway hyper-reactivity as well as extrinsic airway compression7). Intra-luminal bronchial obstruction can result from enlarged bronchial vessels and lymphatics, both of which can result from increased intracardiac filling pressures6). Therefore, extrinsic airway compression and intra-luminal bronchial compression should be considered as causes, in patients with severe respiratory insufficiency or atelectasis following respiratory infection, even if patients have been diagnosed as isolated secundum ASD. For differential diagnosis between extrinsic and intrinsic airway compression, bronchoscopy can be helpful, although it could not be done in this case.
Even a mild degree of extrinsic compression of the bronchus in infants results in airway obstruction because bronchial luminal resistance is inversely proportional to the 4th power of radius of the lumen8,9). Significantly lower small airway obstruction in children with left-to-right shunts correlated with degrees of PAH10). Prolonged extrinsic compression may result in tracheomalacia, bronchomalasia, or both. Pulmonary artery-induced bronchial compression has also been shown to be an important cause of congenial lobar emphysema11). This patient showed improvement of intrinsic bronchial narrowing. If there was not new development of extrinsic airway compression and failure to thrive, we expected to treat the patient by device closure after more weight had been gained. Some reports suggest that device closure of ASD is an effective and fairly safe alternative to surgery in infants12).
In conclusion, in infants with symptomatic isolated ASD, especially those with respiratory insufficiency associated with PAH, evaluation of extrinsic airway compression should be considered. If extrinsic airway compression is not obvious, the intra-luminal bronchial obstruction should be considered as the cause of intrinsic airway compression. These patients may show symptomatic improvement by correction of their congenital heart disease that causes the left-to-right shunt.
References
1. Dickinson DF, Arnold R, Wilkinson JL. Congenital heart disease among 160 480 liveborn children in Liverpool 1960 to 1969: implications for surgical treatment. Br Heart J 1981;46:55–62.



2. Chan KC, Godman MJ, Walsh K, Wilson N, Redington A, Gibbs JL. Transcatheter closure of atrial septal defect and interatrial communications with a new self expanding nitinol double disc device (Amplatzer septal occluder): multicentre UK experience. Heart 1999;82:300–306.



3. Lammers A, Hager A, Eicken A, Lange R, Hauser M, Hess J. Need for closure of secundum atrial septal defect in infancy. J Thorac Cardiovasc Surg 2005;129:1353–1357.


4. Bull C, Deanfield J, de Leval M, Stark J, Taylor JF, Macartney FJ. Correction of isolated secundum atrial septal defect in infancy. Arch Dis Child 1981;56:784–786.



5. Andrews R, Tulloh R, Magee A, Anderson D. Atrial septal defect with failure to thrive in infancy: hidden pulmonary vascular disease? Pediatr Cardiol 2002;23:528–530.


6. Kussman BD, Geva T, McGowan FX. Cardiovascular causes of airway compression. Paediatr Anaesth 2004;14:60–74.


7. Berlinger NT, Long C, Foker J, Lucas RV Jr. Tracheobronchial compression in acyanotic congenital heart disease. Ann Otol Rhinol Laryngol 1983;92(4 Pt 1): 387–390.


8. Corno A, Giamberti A, Giannico S, Marino B, Rossi E, Marcelletti C, et al. Airway obstructions associated with congenital heart disease in infancy. J Thorac Cardiovasc Surg 1990;99:1091–1098.


9. Sebening C, Jakob H, Tochtermann U, Lange R, Vahl CF, Bodegom P, et al. Vascular tracheobronchial compression syndromes: experience in surgical treatment and literature review. Thorac Cardiovasc Surg 2000;48:164–174.


10. Kussman BD, Geva T, McGowan FX. Cardiovascular causes of airway compression. Paediatr Anaesth 2004;14:60–74.


Fig. 1
Chest radiograph at the corrected age of 1 month during the second admission. The patient was intubated, and severe collapse of both lung fields was observed. The diaphragm was flattened, and hyperinflation of both lungs was also observed.
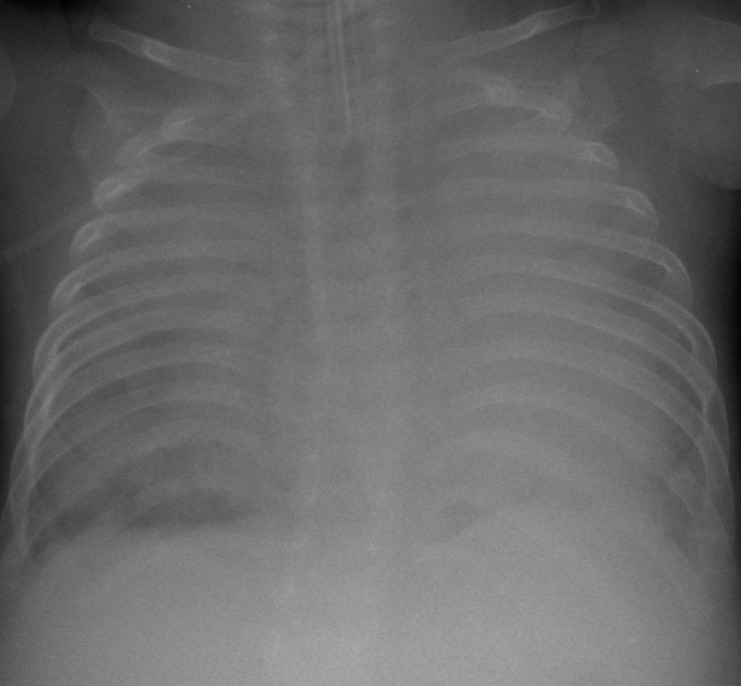
Fig. 2
Computed tomography at the corrected age of 1 month. (A) Collapse in the dependent portion of the right lung and a total collapse in the lower lobe of the left lung are apparent. (B) Coronal image shows narrowing of the main bronchus on the left and the intermediate bronchus on the right. Narrowing of the bronchus was suspected to be because of an intrinsic cause such as bronchomalacia.

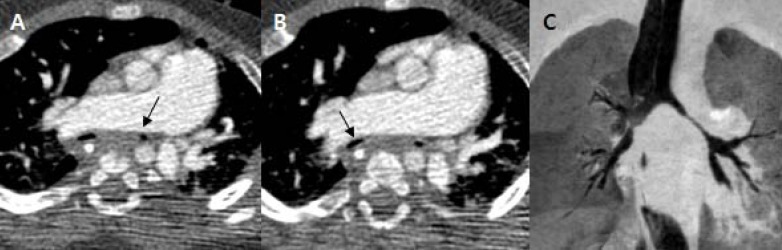
Fig. 3
Preoperative computed tomography at the corrected age of 8 months. Axial image showed dilated pulmonary artery compressing the main bronchus on the left (A, arrow) and the intermediate bronchus on the right (B, arrow). (C) Coronal image shows narrowing of the main bronchus on the left and the intermediate bronchus on the right side.




 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


