Introduction
Pandemic novel infl uenza A (H1N1) virus has quickly spread worldwide following its identifi cation in Mexico in April 2009. The outbreak of H1N1 virus infection resulted in disease burden and mortality in children. Specifically, neurologic complications were more severe compared with seasonal influenza infection, and reached 30% of fl urelated deaths in England1). The incidence of neurologic complications in children was variously reported as 2% in Korea, 6% in England and the United States, and 12% in China1-4). Persistent neurologic symptoms were present in 22% of cases with H1N1 virus infection at the time of hospital discharge compared with, 0% of seasonal influenza cases3-6). The most common neurological complica tions were seizure and encephalopathy. Secondary movement disorders such as chorea, tremor and dystonia were also caused by influenza infection but were very rarely reported7). This study reports a 14yearold boy with secondary paroxysmal kinesigenic dyskinesia (PKD) caused by 2009 H1N1 virus infection.
Case report
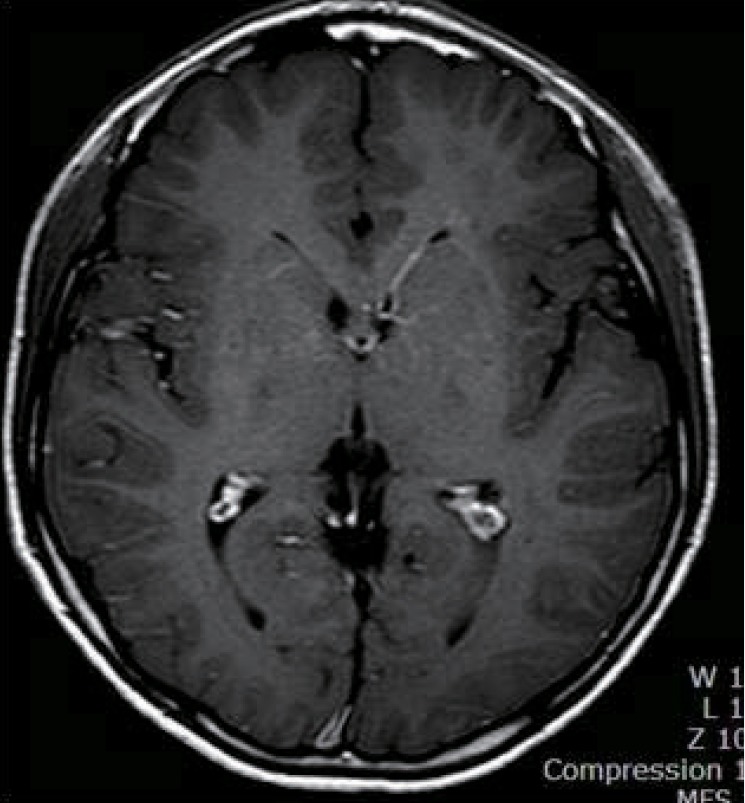
A 14yearold Korean boy was transferred to Inje University Hae undae Paik Hospital with repetitive and paroxysmal seizurelike behavior that persisted for one year. About a year ago, he was taken to the emergency room with respiratory difficulty following fever and cough for 4 days. His respiratory symptoms were progressed to an asthmatic attack and worsened to respiratory failure. Intravenous me thylprednisolone was injected, and a bronchodilator was continuously inhaled. The acute asthmatic at tack was subsequently relieved. His blood pressure was 100/60 mmHg, his heart rate was 92/min, his respiratory rate was 28/min, and his body temperature was 36.5Ōäā. He had no focal neurologic signs and abnormal neurologic examination. The H1N1 virus was confirmed in the respiratory secretion test by real-time polymerase chain reaction. He was administered oseltamivir and discharged by day 8 with no abnormal symptoms. About two weeks after H1N1 virus infection, he showed abnormal behaviors that consisted of indefinable paresthesia of both hands, followed by tonic movement with twisting of both arms and no loss of consciousness. His abnormal behaviors lasted less than one minute and were repeated several times a day. He indicated that the abnormal behaviors were triggered by sudden standing from a sitting position and were aggravated by stress, anxiety, or during outings. His abnormal behaviors led to various physical and psychological traumas. He had normal development without focal neurologic signs and family history of neurologic problems. His electoencephalography (EEG) was normal, and his brain magnetic resonance imaging (MRI) showed an incidental and asymptomatic venous angioma throughout the left basal ganglia extending to the periventricular white matter in the right frontal area (Fig. 1). He had undergone video EEG to differentiate abnormal behaviors from seizures and to obtain a correct diagnosis. He described paresthesialike symptoms to his parents on the brink of total nine each attack lasted less than one minute. The diagnosis was confirmed as PKD associated with H1N1 virus infection. The oral administration of levodopa reduced his symptoms by 90%. His symptoms disappeared completely after adding carbamazepine, but they reappeared after decreasing the dose of levodopa. After that, the patient was being treated with levodopa and carbamazepine, and was symptom free.
Discussion
We describe a 14-year-old boy with secondary PKD caused by 2009 H1N1 infection. Secondary PKD is a rare movement disorder caused by underlying etiology, and is characterized by painless dystonia and/or choreiform movements with brief, frequent episodes triggered by sudden voluntary movements8,9). Secondary movement disorders induced by infection have shorter latency than those caused by other diseases such as stroke, trauma, multiple sclerosis, encephalitis or metabolic abnormalities. The mean latency between the onset of a movement disorder and infection was reported to be 5.9 days6). Cases with secondary PKD following cytomegalovirus encephalitis and streptococcal infection showed latencies as short as one month and one week, respectively as shown in our patient, compared with other underlying disorders at several months to several years7,10).
The pathogenesis for neurologic complications of the H1N1 virus is still not completely understood. However, the some research argues against the possibility of direct brain invasion by the H1N1 virus; in a cerebrospinal fluid study, pleocytosis did not present with seizure and encephalopathy in most cases, and a considerable number of cases showed normal EEG or MRI findings2,3,6,11). Several studies have proposed that H1N1 triggers indirect autoimmune mechanisms, including parainfectious or postinfectious immune reactions or various inflammatory cytokine reactions that damage the blood-brain barrier11). A pattern of brain injury by the H1N1 virus similar to seasonal influenza was observed by microglial activation and immune-mediated reactions in neuropathology12). The H5N1 virus has been reported to induce both short-term and long-term effects in the brain. An increase in activated microglial cells and cytokine expression was characterized in the long-term inflammation addition to the transient loss of dopamine in the substantia nigra and the striatum in mice13). The transient loss of dopamine in the basal ganglia and longterm inflammation by cytokines could induce a vasculopathy such as the asymptomatic venous angioma in our patient. This potential mechanism could be supported by the effectiveness of carbamazepine and levodopa therapy in our patient6,14).
Although secondary PKD may be based on a coincidental relationship with pandemic H1N1 virus infection or its symptoms may be exacerbated by infection in association with other underlying pathology or genetic predisposition, the short latent duration after H1N1 infection and the lack of a family history of movement disorders can let us suggest the possibility of secondary PKD in our patient.
Secondary PKD is characterized by normal neurologic examinations between attacks and brief symptoms, which make a delay in diagnosis likely. Our patient showed brief abnormal behavior, but did not take to evaluate this symptom and resulted in delaying his diagnosis because he was normal between attacks. Once diagnosed, secondary PKD is well controlled and has a better recovery. Therefore, the awareness of H1N1 infection-induced secondary PKD is important to allow for a more timely diagnosis and early intervention in afflicted patients.



 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


