Article Contents
| Korean J Pediatr > Volume 56(1); 2013 |
Abstract
Purpose
Childhood obesity is associated with nonalcoholic fatty liver disease (NAFLD), and it has become one of the most common causes of childhood chronic liver diseases which significant as a cause of liver related mortality and morbidity in children in the United States. The development of simpler and easier clinical indices for medical practice is needed to identify advanced hepatic fibrosis in childhood NAFLD instead of invasive method like liver biopsy. FibroScan and aspartate aminotransferase (AST)-to-platelet ratio index (APRI) have been proposed as a simple and noninvasive predictor to evaluate hepatic fibrosis in several liver diseases. APRI could be a good alternative to detect pathologic change in childhood NAFLD. The purpose of this study is to validate the efficacy of APRI for assessing hepatic fibrosis in childhood NAFLD based on FibroScan.
Methods
This study included 23 children with NAFLD who underwent FibroScan. Clinical, laboratory and radiological evaluation including APRI was performed. To confirm the result of this study, 6 patients received liver biopsy.
Results
Factors associated with hepatic fibrosis (stiffness measurement >5.9 kPa Fibroscan) were triglyceride, AST, alanine aminotransferase, platelet count, APRI and collagen IV. In multivariate analysis, APRI were correlated with hepatic fibrosis (>5.9 kPa). In receiver operating characteristics curve, APRI of meaningful fibrosis (cutoff value, 0.4669; area under the receiver operating characteristics, 0.875) presented sensitivity of 94%, specificity of 66%, positive predictive value of 94%, and negative predictive value of 64%.
The world wide increase in childhood obesity is a significant problem. Obesity is associated with nonalcoholic fatty liver disease (NAFLD), which has become one of the most common causes of childhood chronic liver diseases in the United States1). NAFLD represents a group of diseases ranging from asymptomatic simple fatty liver to nonalcoholic steatohepatitis (NASH) characterized by the histological evidence of apoptosis/inflammation/fibrosis, and to cryptogenic cirrhosis2). NAFLD is also important as a cause of liver-related mortality and morbidity. Therefore, early identification of fibrosis in children is crucial3). Currently, liver biopsy is the gold standard assessment method to investigate hepatic fibrosis and inflammation. However, due to the invasiveness of liver biopsy, it may not be recommended for use in children. Also, it is challenging to use liver biopsy as a reoperation for therapeutic and follow-up purposes4,5). Therefore, an accurate noninvasive procedure to detect the degree of hepatic fibrosis in NAFLD is needed. A noninvasive, rapid, painless, and reproducible technique, transient elastography (FibroScan, Echosens, Paris, France) has been developed to measure liver stiffness6). Liver stiffness and the severity of liver fibrosis in patients with NAFLD have a significant and positive correlation with the results of liver biopsies. Thus, FibroScan can be a useful and objective method to assess the degree of liver fibrosis in NAFLD patients7). However, the high cost of FibroScan equipment and the need of skillful specialists limit its broad use, especially in medical practices8). For this reason, the development of simpler and easier clinical and laboratory indices for medical practice are needed to identify advanced fibrosis in patients with NALFD. The aspartate aminotransferase (AST)-to-platelet ratio index (APRI) has been proposed as a simple and noninvasive predictor to evaluate hepatic fibrosis in several liver diseases9). The assessment predictors of biological liver function include simple blood parameters such as AST and platelets are easy to use and estimate. Therefore, APRI could be a good alternative diagnostic method to FibroScan or liver biopsy8). The purpose of this study is to validate the efficacy of APRI for assessing hepatic fibrosis in childhood NASH based on FibroScan.
We evaluated the clinical and laboratory data of 23 pediatric NASH patients (male/female, 22/1) who underwent FibroScan at Severance Children's Hospital in Seoul, Korea, between March 2008 and February 2011. The diagnosis of NASH was based on: 1) laboratory, radiological, or histopathologic characteristics of steatohepatitis, 2) no history of alcohol consumption, and 3) no other underline liver diseases10).
Weight, height, and body mass index were calculated. Blood test results were obtained after overnight fasting for measurement of serum AST, alanine aminotransferase (ALT), albumin, total bilirubin, platelet count, prothrombin time international normalized ratio, cholesterol, triglyceride (TG), glucose, collagen IV, hyaluronic acid, and AST/ALT ratio (AAR). Ultrasonography (US) and fat magnetic resonance imaging (MRI) quantification of the liver were performed.
The technical instructions of Fibroscan (Ecosens, Paris, France) have been described in detail in our previous reports12). Briefly, an ultrasound transducer probe with an external diameter of 9 mm is mounted on the axis of a vibrator. Vibrations of low amplitude and low frequency (50 Hz) are transmitted by the transducer, which induce an elastic shear wave that propagates through the underlying tissues. Pulse-echo ultrasound acquisition is used to follow the propagation of the shear wave and to measure its velocity, which is directly related to tissue stiffness (the elastic modulus E expressed as E=3ŽüV2, where V is the shear velocity and Žü is the mass density [constant for tissues]): the stiffer the tissue, the faster the shear wave propagates13). Transient elastography measures liver stiffness between 25 mm and 65 mm below the skin surface in a volume that approximates a cylinder that is 1 cm wide and 4 cm long. This volume is at least 100 times larger than a biopsy sample and is, therefore, far more representative of the hepatic parenchyma14). We measured the liver stiffness of the children in our study as reported previously6). In brief, liver stiffness was measured quantitatively on the right lobe of liver through the intercostal spaces with the child in the supine position with maximal abduction of the right arm until 10 successful measurements were obtained. The median value of the 10 measurements of liver stiffness was calculated automatically by software on a microcomputer installed in the FibroScan. The score was expressed as the liver stiffness score in kilopascals (kPa). A success rate for measurement of at least 60% has been considered reliable14,15). However, in our study, patients with a success rate less than 90% were excluded from the analyses in an effort to make the data even more reliable6,16).
Liver biopsy with core biopsy gadget (18-G needle, 150 mm length; Biopince, Amedic, Sollentuna, Sweden) was conducted to obtain liver tissues by our previous study12). Specimens containing at least 10 portal tracts and of at least 15 mm length were used and studied by a pathologist. Biopsy specimens were fixed in formalin and embedded in paraffin. Four-micrometer-thick sections were stained with hematoxylin-eosin-safran, Masson trichrome, and Victoria blue. All specimens were analyzed twice by an experienced liver pathologist blinded to the clinical data. Following the methods of Nobili et al.5), the main histological features commonly described in NALFD/NASH (steatosis, inflammation [portal and lobular], hepatocyte ballooning, and fibrosis) were scored according to the Scoring System for NAFLD recently developed by the National Institutes of Health-sponsored NASH Clinical Research Network and mean NAFLD activity score (NAS) was calculated. The degree of fibrosis was quantified on a 4-point scale: 0, absence of fibrosis; 1, perisinusoidal or portal fibrosis; 2, perisinusoidal and portal/periportal fibrosis; 3, septal or bridging fibrosis; 4, cirrhosis. Liver biopsy was performed in 6 patients.
This study was approved by the Institutional Review Board of Yonsei University College of Medicine, Severance Hospital and carried out in accordance with the Helsinki Declaration (as revised in Edinburgh, 2000). Informed written consent was obtained from all patients or appropriate surrogates prior to study enrollment.
Analysis of the data was performed with PASW ver. 18.0 (IBM Co., Armonk, NY, USA). Univariate analysis was conducted to evaluate the significance of group differences. Logistic regression models were performed for multivariate analysis to identify the most significant correlation among the variables. All variables in univariate analyses with P<0.05 were introduced into the multivariate analysis. The diagnostic performance of the APRI was assessed using receiver operating characteristics (ROC) curves. All cutoff values are associated with the probability of a true positive (sensitivity) and a true negative (specificity). The ROC curve is a plot of sensitivity versus 1-specificity for all possible cutoff values. The most commonly used accuracy index is the area under the ROC curve; values near 1.0 indicate high diagnostic accuracy. ROC curves were constructed for determination of hepatic fibrosis in patient with NAFLD17). Using ROC curves, the optimal cutoff value for APRI was chosen to show a 95% sensitivity, or to obtain a 95% specificity. Furthermore, to optimize the diagnostic performance, the sum of true positives and true negatives over the total number of patients were calculated to maximize the sum of sensitivity and specificity.
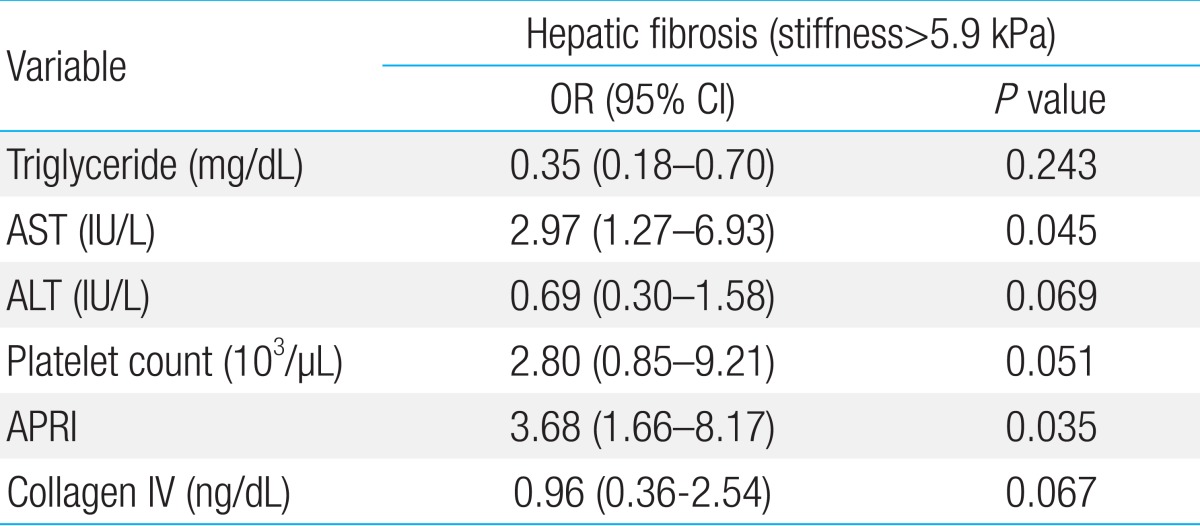
A total of 23 childhood NAFLD patients were included in the investigation. The clinical characteristics of all patients are presented in Table 1. Based on recent studies7), the optimal cutoff value of hepatic fibrosis was set as 5.9 kPa. Sixteen people were in the fibrosis group with liver stiffness greater than 5.9 kPa and 7 people were in the nonfibrosis group with stiffness lesser than 5.9 kPa. There was no gender difference in liver stiffness values. TG, AST, ALT, platelet count, APRI, and collagen IV had significant statistical differences. A multivariate analysis was done using the variable given by the univariate analysis. AST and APRI were correlated with hepatic fibrosis (stiffness greater than 5.9 kPa). TG, ALT, platelet count, and collagen IV had no relationship with hepatic fibrosis (Table 2).
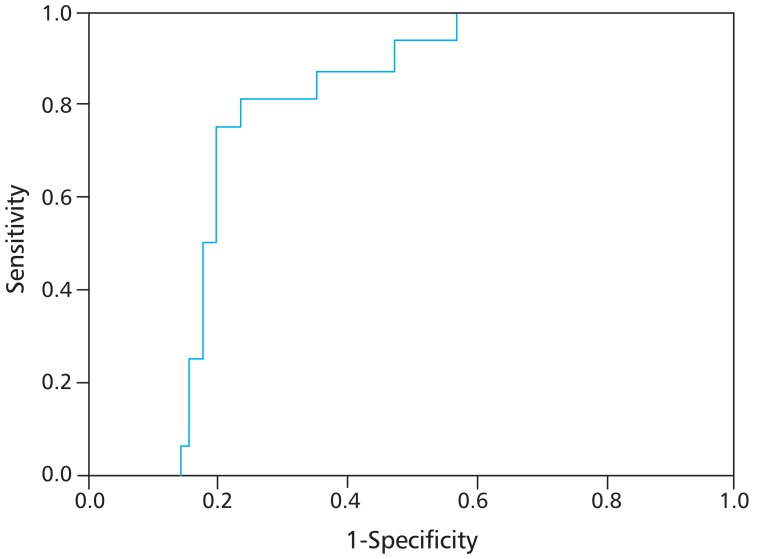
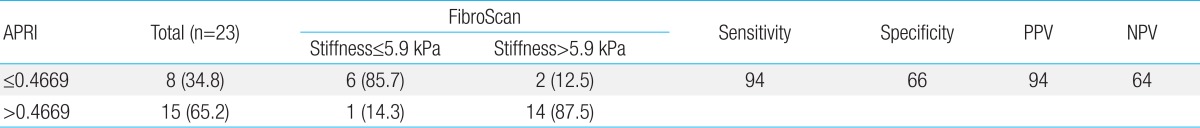
Fig. 1 shows a ROC curve line represented by APRI values of hepatic flexibility and diagnostic accuracy about hepatic fibrosis and cirrhosis. The areas under ROC value according to APRI was 0.875, which show high diagnostic accuracy in liver fibrosis and cirrhosis. To determine the predicting ability of APRI for hepatic fibrosis, an optimal cutoff value was set and sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated (Table 3). Three people (34.8%) were in the group of APRIŌēż0.4669 and 15 people (65.2%) were in the group with APRI>0.4669. The APRI value of meaningful fibrosis, when the cutoff value was set at 0.4669, presented sensitivity of 94%, specificity of 66%, PPV of 94%, and NPV of 64%.
Table 4 shows compared values of APRI of the 6 NAFLD patients who were subjected to liver biopsy tests. For all 6 patients, the FibroScan values were over 8.1 kPa, which was above fibrosis stage one, and APRI value were the same or greater than 0.6531.
Over the past few decades, the prevalence and incidence of obesity in children have increased significantly. Along with this increase, NAFLD has become the most common cause of chronic liver disease in children. Even though the natural history of childhood NAFLD is uncertain, it is suggested that simple steatosis has a benign prognosis, while NASH and fibrosis can develop into cryptogenic cirrhosis and hepatocellular carcinoma1,2). Therefore, early identification of liver fibrosis in NAFLD can aid in prognosis and in determining a therapeutic plan, and it can prevent further development of liver disease17,18). Evaluation of the progression of disease in NAFLD patients depends on serologic tests, US, computed tomography, MRI, and other radioactive test methods. However, fibrosis progression, especially in the early stage, is hard to predict and is often inaccurate.
Until now, liver biopsy has been the best option for evaluating liver fibrosis. It is the best and most objective method of testing, but it is also an invasive method which can bring about a pain and complications, especially in children. Therefore, this method cannot be applied to all children. Also, if enough tissue is not provided, hepatic parenchyma cannot all be represented. In addition, a patient's unwillingness to take the medication and the financial pressure make this method challenging for repetitive medication and further investigation of the patient's condition17,18). Accordingly, research to find a noninvasive and accurate method has been increasing and new techniques are now being invented19,20). Unfortunately, most of the newer methods are expensive or are complicated, and are, therefore, not appropriate or convenient for common usage21,22). Also, chronic hepatitis C (CHC) patients were used in these investigations, and it is uncertain that they can be used childhood NAFLD patients. The recently developed FibroScan is a noninvasive method to measure the liver stiffness14,23). FibroScan has some positive qualities, including a painless procedure, rapid test time (less than 5 minutes), and simple performance. It also covers and evaluates a larger part of the liver and is sensitive compared to the serological markers2). LSM and fibrosis stage in NAFLD patients have a significant correlation, as confirmed by liver biopsy results7). Also, LSM is feasible and related to liver fibrosis in children with chronic liver diseases6). Therefore, LSM is a noninvasive and useful modality for identifying the severity of hepatic fibrosis in childhood NASH patients7). FibroScan determines the degree of liver fibrosis with high accuracy. However, the equipment is expensive and not practical for routine testing in most clinical unit worldwide24). Liver tissue investigation would be much more convenient and financially supportable if a new and simple serologic liver fibrosis method were developed to replace liver biopsy and FibroScan. An ideal serologic fibrosis index which could apply and atypical organ such as liver, must have good repeatability, and low cost. Recently, several studies have attempted to use serologic tests to predict liver fibrosis and cirrhosis25). Most childhood NASH patients that are asymptomatic and have a mild degree of elevated liver enzyme, such as AST and ALT, are followed up without any notifications of the significant hepatic fibrosis in medical practice. Therefore, it is important to introduce simple and predictive serological markers of hepatic fibrosis that can be used in children with NASH at any time.
In this study, FibroScan was used to measure liver stiffness and to overcome the difficulty, limitations, and complications of liver biopsy in children as a baseline study of hepatic fibrosis. The simple and realistic method of APRI as a promising predictor was evaluated for usefulness in measuring liver fibrosis8,26,27). Until now, many investigations have attempted to predict liver fibrosis and cirrhosis through serum markers. However, it is limited due to complex math equations10). Compared to this, APRI is based on two routine laboratory tests and is, therefore, a promising tool with limited expense and widespread availability8). APRI has been used in the evaluation of patients with alcoholic liver fibrosis, CHB, and CHC with high accuracies9). Although the study was performed for CHC, the high diagnostic power of APRI for predicting significant fibrosis has been reported28). Also, relatively high accuracy of APRI for predicting significant fibrosis has been demonstrated even in children with CHB29,30). However, there are few reports using APRI as a noninvasive and useful method for the identification of hepatic fibrosis and cirrhosis in childhood NAFLD. Our study determined the usefulness and reliability of the APRI in assessing fibrosis in children with NAFLD. Yoneda et al.7) used FibroScan and set the liver stiffness cutoff value (kPa) to 5.9. They predicted sensitivity of 86.1%, specificity of 86.1%, PPV of 97.1%, and NPV of 59.3% (which was reasonably important). In this study, data from a previous investigation was used and FibroScan was used to measure the liver flexibility of NAFLD patient. Then, serologic tests were done to determine the correlation between AST and APRI. Based on the above cutoff value of 5.9, when fibrosis (ŌēźF1) was predicted, it was shown that APRI had positive correlation [odds ratio, 3.68; 95% confidence interval, 1.66 to 8.17; P=0.035] with hepatic fibrosis (stiffness>5.9kPa). Commonly, because of the problems and limits of liver biopsies in predicting liver fibrosis, it is effective to use noninvasive serologic markers when area under the ROC (AUROC) values are over 0.8017). AUROC values of APRI also considerably and accurately diagnosed liver fibrosis to those of 0.875. This conveyed superiority in diagnostic usefulness. This did not differ significantly from AUROC values targeted to CHC patients: 0.80, 0.8125,31). This result supported the validness of the recent study. In other studies, APRI has high relationship with liver fibrosis9,26) and one reported high diagnostic accuracy (AUROC, 0.88 [F>2]), 0.94 [F=4])28). Domestically, APRI had a better result as a serologic test indicating liver fibrosis than AAR for CHB patients32). In this study, APRI predictability of fibrosis based on APRI cutoff value of 0.4669 were estimated according to ROC curve results. As a result, high predicted scores (sensitivity of 94%, specificity of 66%, PPV of 94%, and NPV of 64%) have shown that it could be used for predicting liver fibrosis. When 6 NAFLD patients, who have already done liver biopsy, were tested with FibroScan and APRI values, individual FibroScan values were the same or greater than 8.1 kPa, APRI values were the same or greater than 0.6531, and all the patients were above fibrosis stage 1. When an APRI cutoff of 0.4669 was applied, it was applicable in finding out the existence of fibrosis. Therefore, APRI can be used to quickly predict liver fibrosis when liver biopsy is impossible to perform or inapplicable. Although there are several limitations, including the small number of patients and the short follow-up, APRI shows a possibility as a noninvasive method to assess hepatic fibrosis progression. Our results showed that APRI scores correlate significantly to fibrosis in patients with NAFLD. In the future, if object patients increase and colossal cohort study operate and diagnostic accuracy on liver fibrosis is raised by combining serologic test and FibroScan, APRI could be a useful method for predicting liver fibrosis in the early stage, monitoring the process continuously, and determining a therapeutic cure.
In conclusion, this study demonstrated that APRI could be an easy, noninvasive, simple, and readily available method for the prediction of significant hepatic fibrosis in childhood NAFLD. Follow-up evaluation for hepatic fibrosis should be performed by a pediatric hepatologist.
References
1. Barshop NJ, Francis CS, Schwimmer JB, Lavine JE. Nonalcoholic fatty liver disease as a comorbidity of childhood obesity. Ped Health 2009;3:271ŌĆō281.



2. Fierbinteanu-Braticevici C, Dina I, Petrisor A, Tribus L, Negreanu L, Carstoiu C. Noninvasive investigations for non alcoholic fatty liver disease and liver fibrosis. World J Gastroenterol 2010;16:4784ŌĆō4791.



3. Nobili V, Alisi A, Vania A, Tiribelli C, Pietrobattista A, Bedogni G. The pediatric NAFLD fibrosis index: a predictor of liver fibrosis in children with non-alcoholic fatty liver disease. BMC Med 2009;7:21



4. McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010;59:1265ŌĆō1269.


5. Nobili V, Reale A, Alisi A, Morino G, Trenta I, Pisani M, et al. Elevated serum ALT in children presenting to the emergency unit: relationship with NAFLD. Dig Liver Dis 2009;41:749ŌĆō752.


6. de Ledinghen V, Le Bail B, Rebouissoux L, Fournier C, Foucher J, Miette V, et al. Liver stiffness measurement in children using FibroScan: feasibility study and comparison with Fibrotest, aspartate transaminase to platelets ratio index, and liver biopsy. J Pediatr Gastroenterol Nutr 2007;45:443ŌĆō450.


7. Yoneda M, Yoneda M, Mawatari H, Fujita K, Endo H, Iida H, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with nonalcoholic fatty liver disease (NAFLD). Dig Liver Dis 2008;40:371ŌĆō378.


8. Loaeza-del-Castillo A, Paz-Pineda F, Oviedo-Cardenas E, Sanchez-Avila F, Vargas-Vorackova F. AST to platelet ratio index (APRI) for the noninvasive evaluation of liver fibrosis. Ann Hepatol 2008;7:350ŌĆō357.


9. Lin CS, Chang CS, Yang SS, Yeh HZ, Lin CW. Retrospective evaluation of serum markers APRI and AST/ALT for assessing liver fibrosis and cirrhosis in chronic hepatitis B and C patients with hepatocellular carcinoma. Intern Med 2008;47:569ŌĆō575.


10. Fujii H, Enomoto M, Fukushima W, Ohfuji S, Mori M, Kobayashi S, et al. Noninvasive laboratory tests proposed for predicting cirrhosis in patients with chronic hepatitis C are also useful in patients with non-alcoholic steatohepatitis. J Gastroenterol 2009;44:608ŌĆō614.


11. Palmeri ML, Wang MH, Rouze NC, Abdelmalek MF, Guy CD, Moser B, et al. Noninvasive evaluation of hepatic fibrosis using acoustic radiation force-based shear stiffness in patients with nonalcoholic fatty liver disease. J Hepatol 2011;55:666ŌĆō672.



12. Chang HK, Park YJ, Koh H, Kim SM, Chung KS, Oh JT, et al. Hepatic fibrosis scan for liver stiffness score measurement: a useful preendoscopic screening test for the detection of varices in postoperative patients with biliary atresia. J Pediatr Gastroenterol Nutr 2009;49:323ŌĆō328.


13. Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol 2008;48:835ŌĆō847.


14. Castera L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 2005;128:343ŌĆō350.


15. Vizzutti F, Arena U, Romanelli RG, Rega L, Foschi M, Colagrande S, et al. Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology 2007;45:1290ŌĆō1297.


16. Nguyen-Khac E, Chatelain D, Tramier B, Decrombecque C, Robert B, Joly JP, et al. Assessment of asymptomatic liver fibrosis in alcoholic patients using fibroscan: prospective comparison with seven non-invasive laboratory tests. Aliment Pharmacol Ther 2008;28:1188ŌĆō1198.


17. Kim SY, Seok JY, Han SJ, Koh H. Assessment of liver fibrosis and cirrhosis by aspartate aminotransferase-to-platelet ratio index in children with biliary atresia. J Pediatr Gastroenterol Nutr 2010;51:198ŌĆō202.


18. Kaneda H, Hashimoto E, Yatsuji S, Tokushige K, Shiratori K. Hyaluronic acid levels can predict severe fibrosis and platelet counts can predict cirrhosis in patients with nonalcoholic fatty liver disease. J Gastroenterol Hepatol 2006;21:1459ŌĆō1465.


19. Myers RP, Ratziu V, Imbert-Bismut F, Charlotte F, Poynard T. MULTIVIRC Group. Groupe d'Etude Multidisciplinaire sur les Pathologies Li├®es au Virus C. Biochemical markers of liver fibrosis: a comparison with historical features in patients with chronic hepatitis C. Am J Gastroenterol 2002;97:2419ŌĆō2425.


20. Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T, et al. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet 2001;357:1069ŌĆō1075.


21. Afdhal NH, Nunes D. Evaluation of liver fibrosis: a concise review. Am J Gastroenterol 2004;99:1160ŌĆō1174.


22. Poynard T, Imbert-Bismut F, Munteanu M, Messous D, Myers RP, Thabut D, et al. Overview of the diagnostic value of biochemical markers of liver fibrosis (FibroTest, HCV FibroSure) and necrosis (ActiTest) in patients with chronic hepatitis C. Comp Hepatol 2004;3:8



23. Shaheen AA, Wan AF, Myers RP. FibroTest and FibroScan for the prediction of hepatitis C-related fibrosis: a systematic review of diagnostic test accuracy. Am J Gastroenterol 2007;102:2589ŌĆō2600.


24. Zhou K, Gao CF, Zhao YP, Liu HL, Zheng RD, Xian JC, et al. Simpler score of routine laboratory tests predicts liver fibrosis in patients with chronic hepatitis B. J Gastroenterol Hepatol 2010;25:1569ŌĆō1577.


25. Leroy V, Hilleret MN, Sturm N, Trocme C, Renversez JC, Faure P, et al. Prospective comparison of six non-invasive scores for the diagnosis of liver fibrosis in chronic hepatitis C. J Hepatol 2007;46:775ŌĆō782.


26. Shin WG, Park SH, Jang MK, Hahn TH, Kim JB, Lee MS, et al. Aspartate aminotransferase to platelet ratio index (APRI) can predict liver fibrosis in chronic hepatitis B. Dig Liver Dis 2008;40:267ŌĆō274.


27. Borsoi Viana MS, Takei K, Collarile Yamaguti DC, Guz B, Strauss E. Use of AST platelet ratio index (APRI Score) as an alternative to liver biopsy for treatment indication in chronic hepatitis C. Ann Hepatol 2009;8:26ŌĆō31.


28. Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003;38:518ŌĆō526.


29. Wai CT, Cheng CL, Wee A, Dan YY, Chan E, Chua W, et al. Non-invasive models for predicting histology in patients with chronic hepatitis B. Liver Int 2006;26:666ŌĆō672.


30. Lebensztejn DM, Skiba E, Sobaniec-Lotowska M, Kaczmarski M. A simple noninvasive index (APRI) predicts advanced liver fibrosis in children with chronic hepatitis B. Hepatology 2005;41:1434ŌĆō1435.


Fig.┬Ā1
Receiver operating characteristics (ROC) curve for aspartate aminotransferase to platelet ratio index. The area under the ROC curves was 0.875 for the determination of hepatic fibrosis (stiffness>5.9 kPa).
Table┬Ā1
Univariate Analysis for Factors Associated with Hepatic Fibrosis in Childhood Nonalcoholic Steatohepatitis
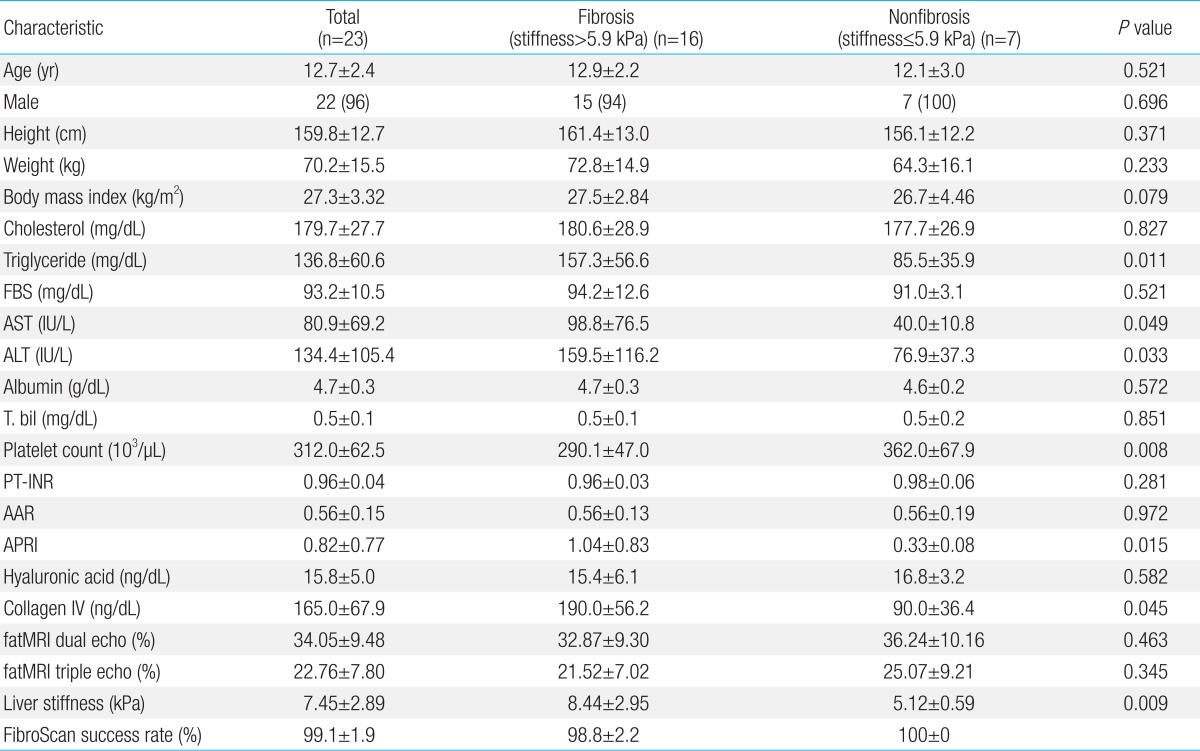
Values are presented as mean┬▒standard deviation or number (%).
FBS, fasting blood sugar; AST, aspartate aminotransferase; ALT, alanine aminotransferase; T. bil, total bilirubin; PT-INR, prothrombin time-international normalized ratio; AAR, AST/ALT ratio; APRI, AST to platelet ratio index; MRI, magnetic resonance imaging.
Table┬Ā2
Multivariate Analysis for Factors Associated with Hepatic Fibrosis in Childhood Nonalcoholic Steatohepatitis




 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


