Introduction
Acute eosinophilic pneumonia is a rare disease that causes an acute onset of respiratory distress and marked pulmonary infiltrates of eosinophils1,2). It presents as acute pneumonia in previously healthy subjects, with possible respiratory failure. Various factors including infections, drugs, toxins, systemic eosinophilic disorders and smoking can cause this disease3,4). Cases in which such causes are not apparent are designated idiopathic acute eosin ophilic pneumonia (IAEP). The main pathologic finding is the presence of organizing diffuse alveolar damage together with marked alveolar and bronchiolar infiltrates of eosinophils1). Approximately two-thirds of patients with IAEP need mechanical ventilation because of acute respiratory distress. However, once the diagnosis is made, complete clinical and radiological recovery without relapse occurs rapidly with corticosteroid therapy3). We present the case of a very young girl who had no apparent history of exposure to the possible causative factors and suffered from acute respiratory distress. After IAEP was confirmed by lung biopsy, she recovered completely in response to corticosteroids without relapse.
Case report
A previously healthy 14-month-old girl was transferred to our hospital because of fever, dry cough and progressive dyspnea for 1 day. On physical examination, she exhibited marked respiratory distress and marked chest wall retractions. Her temperature was 38.6℃, blood pressure was 110/60 mmHg, pulse rate was 180/min, and respiratory rate was 75/min. Her breathing sounds were decreased, and crackles and wheezing were audible in both lungs but without cardiac murmurs.
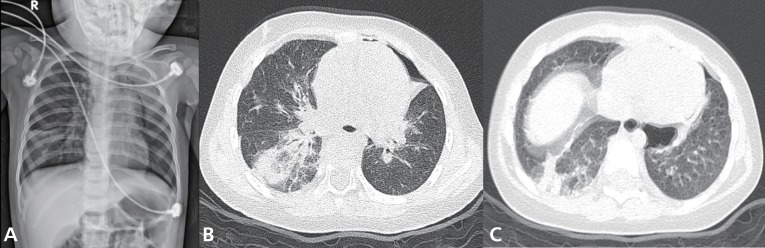
Complete blood cell counts revealed the following: white blood cells, 6,900/uL (neutrophils, 76%; lymphocytes, 19.3%; monocytes, 4.3%; and eosinophils, 0.1%); hemoglobin, 10.7 g/dL; and platelets, 277,000/µL. After 5 L/min of oxygen was applied through a nasal prong, arterial blood gas analysis showed the following: pH, 7.39; PaCO2, 43.5 mmHg; PaO2, 73.7 mmHg; HCO3, 23.9 mEq/L; base excess, -1.6 mEq/L; and oxygen saturation, 94.1%. Other blood biochemical tests were normal. Chest radiography showed patchy consolidations in the right lower lung, bilateral peribronchial infiltrations and pneumothorax in the left upper lung (Fig. 1A). High-resolution computed tomography (CT) of the chest showed multifocal ground-glass opacity, increased interstitial lung markings, parenchymal emphysema and pneumothorax (Fig. 1B, C).
Further workup results were negative for rheumatoid factors, antinuclear antibody, antineutrophil cytoplasmic antibody and fluorescent antinuclear antibody. Serum humoral antibody levels were normal (immunoglobulin [Ig] G, 738.0 mg/dL; IgM, 357.0 mg/dL; IgA, 42.0 mg/dL; and IgE, 11.3 IU/mL). T cell subsets were also within normal limits (lymphocytes, 4,643 /µL; CD4+T cells, 1,518/µL; CD8+T cells, 931/µL; and CD4+/CD8+ T cells, 1.63) and the serum levels of C3, C4, and CH50 were 124 mg/dL, 19.1 mg/dL, and 38.0 U/mL, respectively.
Differential diagnoses, including severe community-acquired pneumonia, acute interstitial pneumonia (AIP), aspiration pneumonia and acute hypersensitivity pneumonitis, were excluded. Blood and sputum cultures for bacteria and fungi were negative. Antibodies for Mycoplasma pneumoniae, Chlamydia trachomatis, Rickettsia tsutsugamushi and Ebstein-Barr virus were negative. Parasitic infestations were excluded by the negative results of tests for specific antibodies for Cysticercus, Sparganum, Paragonimus, and Clonorchis. No respiratory viruses except human bocavirus (HBoV) were detected in the polymerase chain reaction (PCR) of her nasopharyngeal aspirates. Despite broad spectrum antibiotics and supportive care, the patient continued to worsen over the next 48 hours, requiring a positive end-expiratory pressure of 10 cmH2O and a 75% fraction of inspired oxygen to maintain an oxygen saturation of 90%.
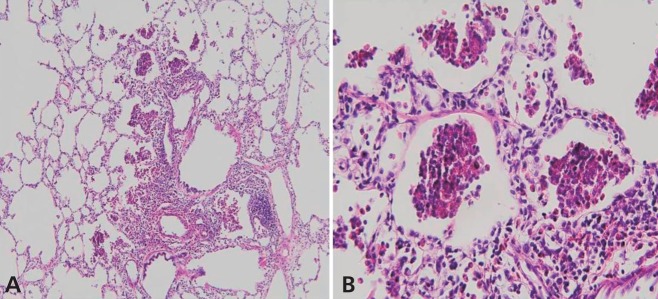
A lung biopsy was performed on the 5th hospital day and demonstrated marked infiltration of eosinophils to alveolar spaces, peribronchiolar tissues and interstitium (Fig. 2A, B). There was no evidence of viral (cytomegalovirus, Epstein-Barr virus, and human immunodeficiency virus), bacterial (Chlamydia trachomatis, Mycoplasma pneumoniae, and Mycobacterium tuberculosis), or fungal infection (Pneumocystis jiroveci) from the lung biopsy specimens.
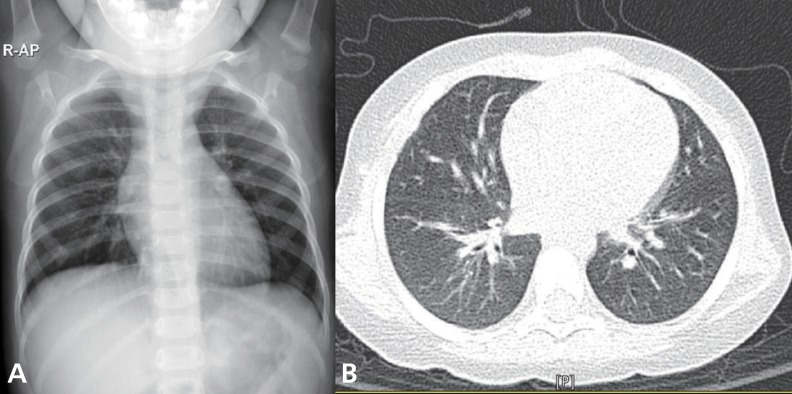
Intravenous corticosteroid pulse therapy (methylprednisolone 30 mg/kg) was begun. The patient's pulmonary status rapidly improved, requiring less ventilator support and showing clearing of infiltrates on chest X-rays (Fig. 3A, B). Three days after the initiation of steroid therapy, the patient was successfully extubated. She was eventually discharged on the 18th day in good condition with a course of oral prednisone therapy tapered for a further 14 days.
Discussion
IAEP rarely occurs in children5). It is mostly manifested by acute onset of fever, dry cough, dyspnea, tachypnea and/or pleural pain. Diagnostic criteria for IAEP are as follows: 1) acute onset of febrile illness (usually less than 7 days), 2) diffuse bilateral pulmonary infiltrates, 3) hypoxemic respiratory failure, 4) lung eosinophilia (bronchoalveolar lavage [BAL] fluid eosinophilia >25% or predominance of eosinophils in lung biopsy), and 5) no known causes of acute eosinophilic lung disease6). The favorable response of IAEP to corticosteroid therapy is characteristic and recurrence is not described3). Our patient met the clinical definition, including the acute onset of fever, respiratory failure and alveolar infiltration of eosinophils without definitive causes. To the best of our knowledge, this is the first case of IAEP in a very young girl.
The causes of eosinophilic pneumonia are various, including smoking, infections, drugs or inhaled agents, and history of dust exposure4,6,7). In our patient, such causes were absent, and we considered it to be idiopathic. HBoV was detected by the PCR of our patient's nasopharyngeal aspirates. Viruses are a rare cause of acute eosinophilic pneumonia. There has been only one report of acute eosinophilic pneumonia which was caused by the 2009 influenza A (H1N1)8). HBoV has not yet been reported as a causative agent of eosinophilic pneumonia. Our patient had cold symptoms 7 days before the onset of clinical symptoms. HBoV infections are ubiquitous and the virus can be detected in the upper airway for up to 2 months following acute infection9). Furthermore, the seroprevalence of HBoV was determined to be up to 58% in children aged <2 years10). It is conceivable that HBoV primarily acts as a silent virus in infants, occasionally causing illnesses rather than being a serious pathogen11). Furthermore, although HBoV related life-threatening respiratory infections were reported12,13), we couldn't confirm their causal relationship in our presented case because viral load was measured neither from respiratory aspirates nor from blood. In addition, reported cases showed neither a lung eosinophilia nor a favorable response to steroid treatment which are distinct characteristics for acute eosinophilic pneumonia. Thus, we thought that the HBoV detected in our patient was a pathogen of the previous upper respiratory infection rather than a causative agent of the current illness. To date, the causes or mechanisms of IAEP are not completely understood, and no single organism or agent has been proved to directly cause acute eosinophilic pneumonia.
Chest radiographs of patients with IAEP show diffuse bilateral infiltrates, airspace opacities, interstitial reticulo-nodular densities and/or pleural effusion. These interstitial infiltrates usually progress to extensive alveolar and interstitial infiltrates involving all lobes in several hours1,2). Chest CT scans show diffuse areas of ground-glass attenuation, alveolar infiltrates, poorly defined nodules and interlobular septal thickening14). These findings of IAEP may mimic patterns seen in patients with congestive heart failure, except that IAEP is not accompanied by cardiomegaly15). The abnormal findings in chest X-rays usually return to normal within 3 weeks of corticosteroid therapy.
Most of the reported cases of IAEP showed, no peripheral blood eosinophilia at presentation, although they showed eosinophilia in BAL fluids or alveolar spaces2,7). Our patient also had extremely low peripheral blood eosinophil counts (0.1% of total white blood cells). Lung biopsy shows acute and organizing diffuse alveolar damage with marked interstitial and alveolar eosinophilic infiltrates, which confirms the disease1,2). Lung biopsy specimen of our patient revealed marked infiltration of eosinophils to peribronchiolar and perivascular tissues of the alveolar spaces as well as the interstitium. Unlike previous cases, alveolar damage was not evident. In the early phase of the disease, edematous alveolar septa with eosinophilic and lymphocytic infiltrations are usually observed, but alveolar damage may not be prominent. As the disease progresses, marked fibrin exudates, hypertrophied type II cells and diffuse alveolar damage become evident16). The pathophysiology of AEP is still not well understood. It appears to result from damage caused by eosinophils infiltrating the lung parenchyma and releasing toxic substances such as basic proteins, lipid mediators, and cytokines3).
Because of the nonspecific signs and symptoms, IAEP is frequently misdiagnosed as severe community-acquired pneumonia, fungal infection or parasitic infestation. Radiologic and histologic features of drug-induced eosinophilic pneumonia are also indistinguishable from IAEP5). Attention must be paid to exposure to various environmental dusts, toxins or cigarette smoke through a detailed history taking and thorough laboratory tests5,6,17).
Two-thirds of IAEP patients progress to respiratory failure, requiring mechanical ventilation3). If the patients are properly treated, their prognoses are excellent. However, without the appropriate diagnosis and treatment, such cases are fatal1). Corticosteroid therapy has been used in most of the reported cases of IAEP. Patients improve significantly within 24 to 48 hours of corticosteroid therapy and are cured without sequelae and recurrence of symptoms within 2 to 12 weeks. Our patient was started on methylprednisolone 30 mg/kg/day. On the third day of treatment, she showed remarkable improvement in clinical symptoms and chest radiographic findings. Two weeks after admission, her chest radiograph showed that infiltrates had resolved, and she did not require supplemental oxygenation. The optimal duration of corticosteroid therapy is uncertain, but 2 to 12 weeks have been reported to be effective. IAEP is a rare disease and has very similar clinical features as AIP and acute respiratory distress syndrome (ARDS)15). However, IAEP must be distinguished from AIP or ARDS because the prognosis of IAEP is excellent so long as corticosteroid therapy is instituted promptly. We reported a rare case of a previously healthy young girl who was diagnosed with IAEP by lung biopsy and recovered completely after corticosteroid therapy. The early diagnosis of IAEP and early start of corticosteroid therapy are essential for lifesaving.



 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


