Article Contents
| Korean J Pediatr > Volume 56(11); 2013 |
Abstract
Purpose
The use of cyclosporine and mini-dose methotrexate (MTX) is a common strategy for graft-versus-host disease (GVHD) prophylaxis in allogeneic transplants. We investigated whether patients who receive fewer than the planned MTX doses are at increased risk for GVHD.
Methods
The study cohort included 103 patients who received allogeneic transplants at the Department of Pediatrics of The Catholic University of Korea College of Medicine, from January 2010 to December 2011. MTX was administered on days 1, 3, 6, and 11 after transplant at a dose of 5 mg/m2 each. Within the cohort, 76 patients (74%) received all 4 doses of MTX [MTX(4) group], while 27 patients (26%) received 0-3 doses [MTX(0-3) group].
Results
Although there was no difference in neutrophil engraftment between the 2 groups, platelet engraftment was significantly faster in the MTX(4) group (median, 15 days), compared to the MTX(0-3) group (median, 25 days; P=0.034). The incidence of grades II-IV acute GVHD was not different between the MTX(4) and MTX(0-3) groups (P=0.417). In the multivariate study, human leukocyte antigen mismatch was the most significant factor causing grades II-IV acute GVHD (P=0.002), followed by female donor to male recipient transplant (P=0.034). No difference was found between the MTX(4) and MTX (0-3) groups regarding grades III-IV acute GVHD, chronic GVHD, and disease-free survival.
Both acute and chronic graft-versus-host disease (GVHD) are significant complications that may occur in children who receive allogeneic hematopoietic stem cell transplantation (HSCT), although the overall incidence is lower than found in the adult patient population1,2). As methods of treating GVHD are limited once it is diagnosed, a significant emphasis is on the prevention of GVHD. Such strategies for prevention may gain greater attention with recent increasing use of unrelated donors and other alternative donors for HSCT.
Since its initial introduction in 1986, a combination of cyclosporine (CsA) and mini-dose methotrexate (MTX) has become an important means of GVHD prophylaxis3,4). This regimen resulted in lower rates of GVHD when compared to prophylaxis with CsA alone in patients who received HSCT for both malignant and nonmalignant disease5,6). Of note, this immunosuppressive combination has also shown efficacy in children who receive allogeneic transplant7).
In this GVHD prophylaxis regimen, 4 doses of MTX are given on days 1, 3, 6, and 11 after transplant. However, 1 or more of these MTX doses are often omitted if the patient shows signs and symptoms of significant mucositis that requires analgesia, or if there is evidence of hepatic or renal toxicity. The overall effect of these omissions on subsequent acute and chronic GVHD incidence remains unclear. An earlier study showed that omission of the final day 11 MTX did not adversely affect incidence of severe acute GVHD and chronic GVHD8), while 2 later reports showed a greater risk for GVHD in patients who were not given all planned doses of MTX9,10). The majority of the patients in these 3 reports were adult recipients of HSCT. As the incidence and severity of GVHD tends to be lower in pediatric patients compared with adults, omissions from the full schedule of prophylactic MTX may result in different outcomes in children.
Our main objective in this study was to compare the incidence of acute and chronic GVHD, and survival between pediatric patients who received the full schedule of mini-dose MTX and patients who were given less than the full schedule, in order to verify the effect of these MTX omissions on important transplant outcomes.
The study cohort included 103 patients who received allogeneic HSCT at the Department of Pediatrics, The Catholic University of Korea College of Medicine during a period of 2 years from January, 2010 to December, 2011 (Table 1).
Donor-recipient pairs were matched at human leukocyte antigen (HLA)-A, B, C, and DRB1 alleles using high resolution typing. Children who were treated for malignant disease received either a total body irradiation (TBI)-based or busulfan-based myeloablative conditioning regimen, while patients who received HSCT for bone marrow failure received a fludarabinecyclophosphamide based conditioning regimen.
Planned GVHD prophylaxis was uniform and consisted of CsA and mini-dose MTX. CsA was started on day-1 intravenously (IV) at a dosage of 3 mg/kg/day, which was subsequently switched to an oral formulation at 5 mg/kg/day once oral intake was possible. Mini-dose MTX was given IV at a dose of 5 mg/m2/day on days 1, 3, 6, and 11 posttransplant. Patients who received transplant from an unrelated donor, haploidentical family donor, or who were transplanted for bone marrow failure received antithymocyte globulin (ATG, Thymoglobulin; Genzyme, Cambridge, MA, USA) (n=85, 83%). ATG was given at a total dose of 7.5 mg/kg as part of the conditioning regimen prior to transplantation, and was increased to 10 mg/kg for recipients of haploidentical transplants. One or more of the MTX doses was omitted if the patient showed signs of mucositis that required narcotic analgesia, at the discretion of the physician responsible for each respective patient. Antimicrobial prophylaxis was done as previously published11).
Posttransplantation outcomes that were evaluated included neutrophil and platelet engraftment, acute GVHD grades II-IV, acute GVHD grades III-IV, chronic GVHD, disease-free survival (DFS), and overall survival (OS). Date of neutrophil engraftment was defined as the first of three consecutive days with absolute neutrophil count>1.0├Ś109/L, and the date of platelet engraftment was defined as the first of three consecutive days with platelet count>50├Ś109/L without platelet transfusions in the preceding week. Acute and chronic GVHD were diagnosed and graded according to previously published criteria12,13).
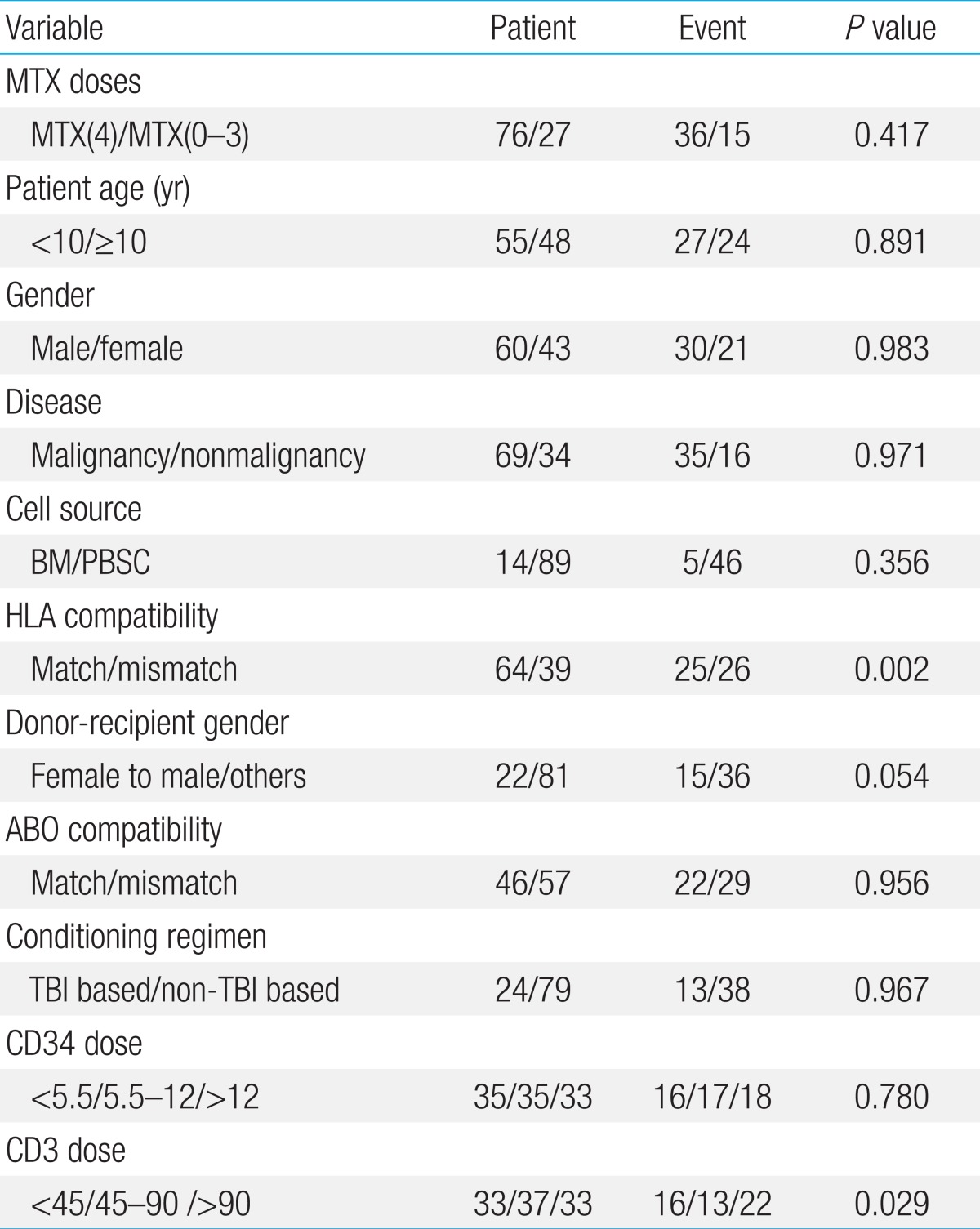
The following variables were studied for possible predictive value with regards to acute and chronic GVHD: number of MTX doses infused (4 doses of MTX [MTX(4)] vs. 0-3 doses of MTX [MTX(0-3)]), patient age (<10 years old vs. Ōēź10 years old), and gender, underlying disease (malignancy vs. nonmalignant disease), donor-recipient HLA compatibility (full match vs. mismatch), cell source (bone marrow vs. peripheral blood stem cells), conditioning regimen (TBI based vs. non-TBI based), donor-recipient gender (female to male vs. others), ABO compatibility, and infused CD34+ and CD3+ cell dose divided into 3 equal groups. In addition, antecedent acute GVHD was studied for prognostic significance with regards to chronic GVHD.
Comparison of neutrophil and platelet engraftment between the MTX(4) and MTX(0-3) groups was done with the Mann-Whitney test. The impact of independent variables on acute and chronic GVHD was done with the cumulative incidence function and Gray test for univariate study. For acute GVHD, death before day 100 was considered a competing risk, while for chronic GVHD death before 1 year was considered a competing risk. Multivariate study was done with factors having P<0.1 in univariate study, and was calculated according to Fine and Gray semiparametric proportional hazards model14). DFS and OS were calculated using the Kaplan-Meier method and comparisons made with the log-rank test. Patients alive were censored at date of last follow-up, but no later than April 30th, 2013. SPSS ver. 16 (SPSS Inc., Chicago, IL, USA), and R package ver. 2.14.2 (R foundation for Statistical Computing, Vienna, Austria). P<0.05 were considered statistically significant.
Within the study cohort, 76 patients (74%) received all MTX(4) while 27 patients (26%) received MTX(0-3). Of the patients who received less than the scheduled MTX doses, 19 patients received 3 doses (18%), 4 patients received 2 doses (4%), 3 patients received 1 dose (3%), and 1 patient did not receive MTX (1%). The reason for omission of scheduled MTX in these patients was severe oral mucositis in all cases.
The medians of infused cell counts were as follows: TNC 16.7├Ś108/kg (range, 1.4-89.7├Ś108/kg), MNC 11.4├Ś108/kg (range, 0.5-52.6├Ś108/kg), CD34+ 7.8├Ś106/kg (range, 1.6-53.4├Ś106/kg), and CD3+ 63.8├Ś107/kg (range, 1.6-284.3├Ś107/kg). All patients showed neutrophil engraftment at a median of 12 days (range, 9-27 days), while 96 patients (93%) showed platelet engraftment at a median of 17 days (range, 7-258 days). No difference in time to neutrophil engraftment was observed between the MTX(4) and MTX(0-3) groups (median, 12 days for both groups), while platelet engraftment was significantly faster at a median of 15 days in the MTX(4) group, compared to 25 days for the MTX(0-3) group (P=0.034) (Table 2).
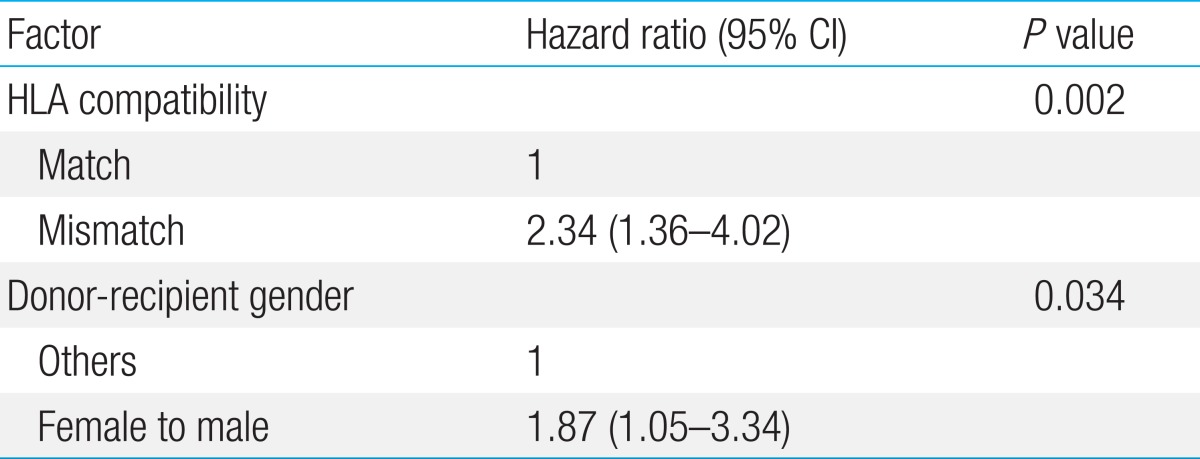
Overall cumulative incidence of grades II-IV acute GVHD was 49.5%┬▒5.0% (51/103). In univariate study, HLA mismatch (P=0.002), female donor to male recipient transplant (P=0.054), and higher infused CD3+ cell dose (P=0.029) proved to be important (Table 3). In contrast, the number of MTX doses infused did not have significance with regards to grades II-IV acute GVHD (P=0.417). In multivariate study, HLA compatibility was the most significant factor for predicting grades II-IV acute GVHD (P=0.002) followed by donor-recipient gender (P=0.034) (Table 4).
Overall incidence of severe acute GVHD (grades III-IV) was 7.8%┬▒2.7% (8/103), including 6 patients with grade III and 2 patients with grade IV acute GVHD. None of the studied factors proved to be significant with regards to predicting grades III-IV acute GVHD. Specifically, the doses of MTX infused did not have an impact on grades III-IV acute GVHD (P=0.429). One of the 8 patients died of causes related to GVHD.
The incidence of chronic GVHD was 29.1%┬▒4.5% (30/103), including 4 patients with limited and 26 patients with extensive chronic GVHD. In univariate study, recipients of 0-3 doses of MTX (P=0.034), female gender (P=0.072), malignant disease (P=0.074), and preceding acute GVHD (P=0.098) were important factors for chronic GVHD incidence. However, none of these factors were significant in multivariate study. None of the patients died from chronic GVHD-related causes in underlying disease remission.
The DFS for the cohort was 57.6%┬▒5.4% (63/103). No difference in DFS was found between the MTX(4) and MTX(0-3) groups (56.7%┬▒6.3% and 62.6%┬▒9.4% respectively, P=0.939) (Fig. 1).
The OS for the cohort was 68.8%┬▒5.2% (74/103). Twenty-five patients (24%) died in relapsed disease state. The cause of nonrelapse death for the remaining 4 patients was as follows: acute GVHD (n=1), cytomegalovirus encephalitis and cerebral aspergillosis (n=1), hepatic veno-occlusive disease (n=1), and acute respiratory distress syndrome (n=1).
Although MTX infusion posttransplantation has been linked to delayed engraftment15), we found no difference in neutrophil engraftment between the MTX(4) and MTX(0-3) groups in our study. Furthermore, platelet engraftment was significantly faster in recipients of the complete MTX schedule compared with patients who received less than four doses. The reasons for the disparity in platelet engraftment are not clear, but the severe oral mucositis, which was a common characteristic of the MTX(0-3) group may have led to other posttransplant complications that resulted in delayed platelet recovery.
Our results indicate that infusion of less than the complete schedule of mini-dose MTX has no effect on outcomes such as acute and chronic GVHD, and survival. One or more of the doses of MTX are often omitted due to severe mucositis occurring after conditioning, or due to hepatic or renal impairment during this period. Oral mucositis is a major complication of the immediate posttransplantation period that is both difficult to treat and is a significant impediment to adequate enteral intake16,17). As MTX infusion itself may cause or aggravate mucositis18), continued adherence to the full MTX dosing schedule is difficult in patients with signs and symptoms of severe mucositis. We conclude that in such situations doses of MTX may be omitted to allow for rapid improvement of mucositis without untoward effects on either GVHD or survival.
In our study, the overall incidence of grades II-IV acute GVHD (49.5%) was significantly higher than has been reported for pediatric transplant cohorts19). The reasons for the increased rate of acute GVHD are not clear, but the large number of alternative donor transplants that were undertaken most likely contributed to this increased incidence. Donor-recipient HLA compatibility proved to be an important factor for acute GVHD incidence, as has been reported previously20). Increased rate of chronic GVHD in female donor-male recipient transplants has been reported21), and the importance of this specific gender disparity as a factor for acute GVHD in our study underscores its potential role as a risk factor for GVHD overall. Despite the high rate of grades II-IV acute GVHD, the rate of grades III-IV GVHD which are more resistant to treatment was low (7.8%), so that we were unable to conclude upon significant predictors for this more problematic type of GVHD.
The major limitations of our study are as follows; first, the number of patients who did not receive all 4 MTX doses was much less than patients who completed scheduled dosing. As a result, we were unable to divide the patient cohort according to the specific number of MTX doses received, but combined these patients into one group. Much of previous literature on this topic focused on differences in outcome when the final fourth MTX dose was omitted only, with one study concluding on no difference in incidence of acute GVHD grades II-IV and chronic GVHD between patients who received the complete MTX schedule and patients in whom the fourth dose was omitted8), while another study found a greater risk for severe acute GVHD (grades III-IV) in patients who did not receive the fourth dose of MTX9). In our study, as all patients who received less than the complete schedule were placed in one group, the effect of a specific MTX omission on outcomes remains unclear. Finally, we stress that the majority of patients received ATG as part of the conditioning regimen, and its use may have affected GVHD incidence. Recent studies indicate that in vivo T-cell depletion using ATG is effective in preventing GVHD in children who receive unrelated transplants22,23). Hence, the comparable outcomes observed between the MTX(4) and MTX(0-3) groups in our study may have been influenced by the use of ATG, and the results herein may be limited to the setting of ATG-based T-cell depletion. A question to pursue, then, is whether CsA alone is sufficient for GVHD prophylaxis when ATG is also used, a notion which is contrary to at least 1 study which showed that a 1 drug regimen of prophylaxis (that is, either CsA or MTX) is a risk factor for severe acute GVHD in children24).
In conclusion, our study in a cohort of children who received allogeneic transplant indicates that omissions from the full dose of mini-MTX for GVHD prophylaxis due to severe patient oral mucositis are not detrimental in terms of either GVHD incidence or survival. These results should aid in clinical decision making with regards to MTX dosing, and possibly allow for faster patient recovery from oral mucositis and lower mucositis-related morbidity.
References
1. Eapen M, Horowitz MM, Klein JP, Champlin RE, Loberiza FR Jr, Ringden O, et al. Higher mortality after allogeneic peripheral-blood transplantation compared with bone marrow in children and adolescents: the Histocompatibility and Alternate Stem Cell Source Working Committee of the International Bone Marrow Transplant Registry. J Clin Oncol 2004;22:4872ŌĆō4880.


2. Zecca M, Prete A, Rondelli R, Lanino E, Balduzzi A, Messina C, et al. Chronic graft-versus-host disease in children: incidence, risk factors, and impact on outcome. Blood 2002;100:1192ŌĆō1200.


3. Storb R, Deeg HJ, Whitehead J, Appelbaum F, Beatty P, Bensinger W, et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med 1986;314:729ŌĆō735.


4. Storb R, Deeg HJ, Pepe M, Appelbaum F, Anasetti C, Beatty P, et al. Methotrexate and cyclosporine versus cyclosporine alone for prophylaxis of graft-versus-host disease in patients given HLA-identical marrow grafts for leukemia: long-term follow-up of a controlled trial. Blood 1989;73:1729ŌĆō1734.


5. Ringden O, Horowitz MM, Sondel P, Gale RP, Biggs JC, Champlin RE, et al. Methotrexate, cyclosporine, or both to prevent graft-versus-host disease after HLA-identical sibling bone marrow transplants for early leukemia? Blood 1993;81:1094ŌĆō1101.


6. Iravani M, Mousavi A, Gholibeikian S, Bahar B, Samiee S, Ashouri A, et al. Cyclosporin A and mini short-term methotrexate vs cyclosporin A as graft-versus-host disease prophylaxis in patients with beta thalassemia major undergoing allogeneic blood and marrow transplantation. Bone Marrow Transplant 2005;35:1095ŌĆō1099.


7. Storb R, Sanders JE, Pepe M, Anasetti C, Appelbaum FR, Buckner CD, et al. Graft-versus-host disease prophylaxis with methotrexate/cyclosporine in children with severe aplastic anemia treated with cyclophosphamide and HLA-identical marrow grafts. Blood 1991;78:1144ŌĆō1145.


8. Atkinson K, Downs K. Omission of day 11 methotrexate does not appear to influence the incidence of moderate to severe acute graft-versus-host disease, chronic graft-versus-host disease, relapse rate or survival after HLA-identical sibling bone marrow transplantation. Bone Marrow Transplant 1995;16:755ŌĆō758.

9. Kumar S, Wolf RC, Chen MG, Gastineau DA, Gertz MA, Inwards DJ, et al. Omission of day +11 methotrexate after allogeneic bone marrow transplantation is associated with increased risk of severe acute graft-versus-host disease. Bone Marrow Transplant 2002;30:161ŌĆō165.


10. Medd P, Monk I, Danby R, Malladi R, Clifford R, Ellis A, et al. Methotrexate dose delivery is more important than ciclosporin level in graft-versus-host disease prophylaxis following T-replete reduced-intensity sibling allogeneic stem cell transplant. Int J Hematol 2011;94:266ŌĆō278.


11. Chung NG, Lee JW, Jang PS, Jeong DC, Cho B, Kim HK. Reduced dose cyclophosphamide, fludarabine and antithymocyte globulin for sibling and unrelated transplant of children with severe and very severe aplastic anemia. Pediatr Transplant 2013;17:387ŌĆō393.


12. Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995;15:825ŌĆō828.

13. Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant 2003;9:215ŌĆō233.


14. Scrucca L, Santucci A, Aversa F. Regression modeling of competing risk using R: an in depth guide for clinicians. Bone Marrow Transplant 2010;45:1388ŌĆō1395.


15. Cutler C, Kim HT, Hochberg E, Ho V, Alyea E, Lee SJ, et al. Sirolimus and tacrolimus without methotrexate as graft-versus-host disease prophylaxis after matched related donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant 2004;10:328ŌĆō336.


16. Gori E, Arpinati M, Bonifazi F, Errico A, Mega A, Alberani F, et al. Cryotherapy in the prevention of oral mucositis in patients receiving low-dose methotrexate following myeloablative allogeneic stem cell transplantation: a prospective randomized study of the Gruppo Italiano Trapianto di Midollo Osseo nurses group. Bone Marrow Transplant 2007;39:347ŌĆō352.


17. Bechard LJ, Guinan EC, Feldman HA, Tang V, Duggan C. Prognostic factors in the resumption of oral dietary intake after allogeneic hematopoietic stem cell transplantation (HSCT) in children. JPEN J Parenter Enteral Nutr 2007;31:295ŌĆō301.



18. Cutler C, Li S, Kim HT, Laglenne P, Szeto KC, Hoffmeister L, et al. Mucositis after allogeneic hematopoietic stem cell transplantation: a cohort study of methotrexate- and non-methotrexate-containing graft-versus-host disease prophylaxis regimens. Biol Blood Marrow Transplant 2005;11:383ŌĆō388.


19. Lin YF, Lairson DR, Chan W, Du XL, Leung KS, Kennedy-Nasser AA, et al. Children with acute leukemia: a comparison of outcomes from allogeneic blood stem cell and bone marrow transplantation. Pediatr Blood Cancer 2011;56:143ŌĆō151.


20. Flomenberg N, Baxter-Lowe LA, Confer D, Fernandez-Vina M, Filipovich A, Horowitz M, et al. Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood 2004;104:1923ŌĆō1930.


21. Kondo M, Kojima S, Horibe K, Kato K, Matsuyama T. Risk factors for chronic graft-versus-host disease after allogeneic stem cell transplantation in children. Bone Marrow Transplant 2001;27:727ŌĆō730.


22. Ka┼éwak K, Porwolik J, Mielcarek M, Gorczynska E, Owoc-Lempach J, Ussowicz M, et al. Higher CD34(+) and CD3(+) cell doses in the graft promote long-term survival, and have no impact on the incidence of severe acute or chronic graft-versus-host disease after in vivo T cell-depleted unrelated donor hematopoietic stem cell transplantation in children. Biol Blood Marrow Transplant 2010;16:1388ŌĆō1401.


Fig.┬Ā1
Disease-free survival (DFS) according to the number of mini-methotrexate (MTX) doses infused. The bold line shows DFS for recipients of all 4 doses. Dashed line shows the DFS for recipients of 0-3 doses.

Table┬Ā1
Main characteristics of the study cohort (n=103)

Values are presented as number (%) unless otherwise indicated.
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CML, chronic myeloid leukemia; JMML, juvenile myelomonocytic leukemia; MDS, myelodysplastic syndrome; NHL, non-Hodgkin lymphoma; HLH, hemophagocytic lymphohistiocytosis; CAEBV, chronic active Epstein-Barr virus infection; LCS, Langerhans cell sarcoma; SAA, severe aplastic anemia; FA, Fanconi anemia; CDA, congenital dyserythropoietic anemia; KD, Kostmann disease; BM, bone marrow; PBSC, peripheral blood stem cells; HLA, human leukocyte antigen; TBI, total body irradiation; ATG, antithymocyte globulin; MTX, methotrexate.




 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


