Article Contents
| Korean J Pediatr > Volume 58(1); 2015 |
|
Abstract
Group D streptococci are known to cause newborn septicemia and meningitis, but the Streptococcus bovis group strains rarely cause serious neonatal infections in Korea. Central nervous system (CNS) complications of neonatal S. bovis group infection have rarely been reported. In adults, S. bovis group strains cause bacteremia and endocarditis, and are associated with gastrointestinal malignancy. However, only a few studies have reported meningitis and septicemia in infants. Here, we describe a case of bacteremia and meningitis due to Streptococcus gallolyticus subsp. pasteurianus with a delayed CNS complication in an infant. A 28-day-old male infant was admitted to the hospital with a 1-day history of fever. Cultures of blood, cerebrospinal fluid, and urine showed the presence of S. bovis group strain-S. gallolyticus subsp. pasteurianus. He was discharged after 21 days of intravenous ampicillin and cefotaxime administration. Two weeks later, he was readmitted with a fever and short episodes of tonic-clonic movements. Brain magnetic resonance imaging showed marked bilateral frontal subdural effusion. He was discharged after 31 days of antibiotic therapy, and no neurological sequelae were observed at the 9-month follow-up. In conclusion, we present a rare case of neonatal S. gallolyticus subsp. pasteurianus infection causing urinary tract infection, septicemia, meningitis, and delayed CNS complications. This case emphasizes the need for physicians to be aware of S. bovis infection in infants.
Although numerous cases of serious neonatal infection by Lancefield streptococcal groups A, B, C, D, F, and G have been reported, there have been few reports of serious neonatal infection caused by nonenterococcal group D streptococci such as Streptococcus bovis group strains1). In adults, S. bovis group strains are known to cause bacteremia and endocarditis, and are associated with gastrointestinal malignancy2,3,4). There have been sporadic reports of meningitis and septicemia caused by S. bovis group strains in young infants5,6,7). We report a case of late-onset neonatal S. bovis meningitis and septicemia with central nervous system complications.
A 28-day-old male infant was admitted to Korea University Ansan Hospital with a 1-day history of fever, lethargy, and moaning sounds. He was born at term (gestational age 38 weeks and 4 days, weight 3,600 g) via vaginal delivery after an uncomplicated pregnancy. He had been in good health until he developed a fever of 38.0℃ the day before admission. Physical examination was unremarkable except for a body temperature of 38.7℃. He was alert with an open and flat anterior fontanelle, normal grasp reflexes, normal neuromuscular tone, no neck stiffness, and no skin eruptions. Chest radiography findings were normal. His peripheral white blood cell (WBC) count was 5,120/dL (neutrophils 46.5%, lymphocytes 40.4%, monocytes12.7%) and his C-reactive protein level was 13.48 mg/dL. A lumbar puncture was performed and cerebrospinal fluid (CSF) analysis showed marked pleocytosis with a WBC count of 4,000/µL (neutrophils 78%, lymphocytes 8%), red blood cell (RBC) count of 440/µL, protein concentration of 319 mg/dL, and glucose concentration of 4 mg/dL. The CSF/serum glucose ratio was 0.03 (normal value 0.6). Cultures of blood, CSF, and urine obtained by bladder puncture grew S. bovis group strains on day 5, despite absence of pyuria. Empirical antibiotic treatment was initiated immediately after admission with intravenous ampicillin 300 mg/kg/day and cefotaxime 200 mg/kg/day. The cultured bacteria were sensitive to penicillin, cefotaxime, and clindamycin. Amplification and sequencing of ribosomal RNA identified S. gallolyticus subsp. pasteurianus.
The patient was afebrile from day 5 and received a total of 21 days of intravenous ampicillin and cefotaxime. CSF analysis on days 4 and 12 showed low WBC count of 130 cells/µL and 15 cells/µL, respectively. Repeat bacterial cultures of blood, CSF, and urine grew no pathogens. He was discharged on day 21 with a bilateral reduction in visual evoked potentials, but no empyema or subdural effusion on cranial ultrasonography.
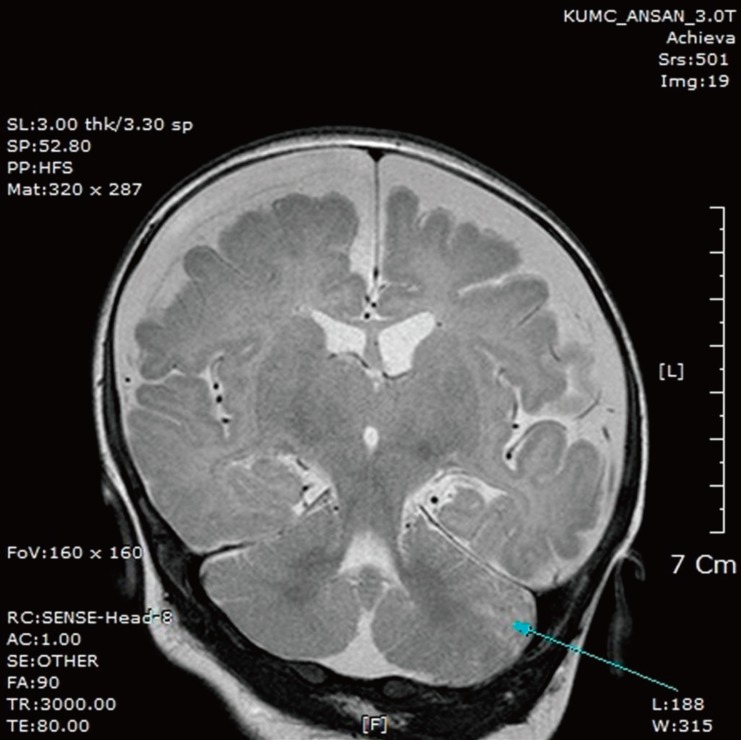
Two weeks after discharge, the patient was rehospitalized with a 2-day history of fever and a 1-day history of seizure activity. He had a mild fever with a maximum temperature of 37.8℃. Several episodes of tonic-clonic movements of the left arm and leg were observed, each lasting a few seconds. A lumbar puncture was performed, and CSF analysis showed pleocytosis with a WBC count of 70/µL (neutrophils 11%, lymphocytes 31%, mononuclear cells 56%), RBC count of 970/µL, protein concentration of 339 mg/dL, and glucose concentration of 33 mg/dL. The CSF/serum glucose ratio was 0.3. Brain magnetic resonance imaging showed marked bilateral frontal subdural effusion (Fig. 1). Ultrasound-guided needle aspiration of the effusion was performed on days 6 and 20. Analysis of the aspirated fluid on day 6 showed pleocytosis with a WBC count of 780/µL, RBC count of >10,000/µL, protein concentration of 2,564 mg/dL, and glucose concentration of 70 mg/dL. On day 20, the CSF WBC count was 15/µL, RBC count was >10,000/µL, protein concentration was 2,944 mg/dL, and glucose concentration was 83 mg/dL. Bacterial cultures of the aspirated effusion grew no pathogens. He was discharged after 31 days of intravenous ampicillin and cefotaxime. At follow-up after 9 months, his visual evoked potentials were improved and no neurological sequelae were observed.
S. bovis group strains are gram-positive bacteria that form part of the normal colonic flora in some individuals. These organisms are most recognized for causing endocarditis in adults, and their association with colonic neoplasms3). Sporadic cases of invasive infection with S. bovis group strains have been described in young infants and neonates8).
Group D streptococci are well known to cause newborn septicemia and meningitis, especially Enterococcus spp.5). In contrast, S. bovis group strains are uncommon neonatal pathogens. Headings et al.5) first described S. bovis infection in a neonate in 1978. A literature search produced only six reported cases of nonenterococcal group D streptococcal meningitis in the English literature from the late 1980s to the 2000s9). From 2000 to 2011, eight cases of S. bovis meningitis were reported in the English literature10). To our knowledge, this is the first report of S. bovis septicemia and meningitis in a Korean neonate or infant. The frequency of S. bovis infection may be underestimated, because S. bovis can be mislabeled as enterococci or viridians group streptococci.
Central nervous system complications of S. bovis group infection have rarely been reported. Klatte et al.11) reported four infants with meningitis caused by S. gallolyticus subsp. pasteurianus. Although two of these infants presented with seizure-like activity, there were no neurological sequelae. Neurological complications are rare in adult cases of S. bovis meningitis12). Only one adult case of S. bovis meningitis with neurological complications has been reported, in a 70-year-old alcoholic with underlying central nervous system disease, who developed subdural empyema after 14 days of treatment with penicillin G. In our case, the patient developed seizure activity after 21 days of antibiotic therapy because of subdural effusion. These cases indicate that S. bovis group meningitis may have some similar presentations to group B streptococcal meningitis, such as delayed subdural effusion. S. bovis generally seems to be sensitive to penicillin, and neither abscess nor empyema formation occurred.
Neonatal S. bovis group infection has a similar clinical presentation to group B streptococcal infection5). Bacteremia is the most common clinical manifestation of early-onset S. bovis group infection, with meningitis being less common8). Neonates with early-onset S. bovis group bacteremia generally present with acute onset of respiratory distress and sepsis within the first 5 days of life1). In contrast, late-onset S. bovis group infection generally presents with urinary sepsis or meningitis9). Early-onset S. bovis infection might result from the intrapartum transmission of bacteria2), but the pathogenesis of late-onset invasive S. bovis infection in infants is unclear. Fikar and Levy1) reported that the pathogenic organism infecting their patient was also grown from rectal and vaginal cultures from the patient's mother. Unfortunately, we did not collect samples for bacterial culture from our patient's mother.
S. bovis group bacteria include two biotypes: S. bovis I (S. gallolyticus) and S. bovis variant or S. bovis II. S. bovis II includes two sub-biotypes: S. bovis II/1 (S. infantarius) and S. bovis II/2 (S. pasteurianus)13). Preliminary studies of the S. bovis biotypes isolated from patients suggest that specific biotypes are associated with specific clinical manifestations8,11). Ruoff et al.14) demonstrated that S. bovis I was most often associated with endocarditis and malignant or premalignant colonic lesions. In contrast, S. bovis II was associated with meningitis or neonatal infection. The reasons for these differences may include virulence factors of the specific organisms, host susceptibility, and differences in maternal colonization. Kim et al.3) reported a patient with infective endocarditis caused by S. bovis I (S. gallolyticus subsp. gallolyticus) and underlying colon cancer, Jeong et al.15) reported a case of severe septic shock caused by S. bovis II/2 (S. gallolyticus subsp. pasteurianus) infection in an adult, and Onoyama et al.8) described neonatal bacteremia and meningitis caused by S. bovis II/2 infection. We performed biotyping of the organism cultured from our patient by amplification and sequencing of the ribosomal RNA, and identified it as S. bovis biotype II/2. The differentiation of biotypes in the S. bovis group may provide a useful predictor of disease progression.
S. bovis group infection appears to have a relatively good prognosis and a low mortality rate9). Although our patient developed delayed-onset subdural effusion and bilateral reduction of visual evoked potentials, subsequent follow-up did not reveal any neurological sequelae. His cognitive and developmental milestones will be followed up for several years.
In conclusion, we report a complicated case of neonatal S. gallolyticus subsp. pasteurianus infection causing urinary tract infection, septicemia, and meningitis.
References
1. Fikar CR, Levy J. Streptococcus bovis meningitis in a neonate. Am J Dis Child 1979;133:1149–1150.


2. Corredoira J, Alonso MP, Coira A, Casariego E, Arias C, Alonso D, et al. Characteristics of Streptococcus bovis endocarditis and its differences with Streptococcus viridans endocarditis. Eur J Clin Microbiol Infect Dis 2008;27:285–291.


3. Kim SY, Joo SI, Yi J, Kim EC. A case of Streptococcus gallolyticus subsp. gallolyticus infective endocarditis with colon cancer: identification by 16S ribosomal DNA sequencing. Korean J Lab Med 2010;30:160–165.


4. Boleij A, Schaeps RM, Tjalsma H. Association between Streptococcus bovis and colon cancer. J Clin Microbiol 2009;47:516



5. Headings DL, Herrera A, Mazzi E, Bergman MA. Fulminant neonatal septicemia caused by Streptococcus bovis. J Pediatr 1978;92:282–283.


6. Gavin PJ, Thomson RB Jr, Horng SJ, Yogev R. Neonatal sepsis caused by Streptococcus bovis variant (biotype II/2): report of a case and review. J Clin Microbiol 2003;41:3433–3435.



7. Okumura A, Takahashi H, Ogawa A, Kuno K, Watanabe K. Streptococcus bovis meningitis in an otherwise healthy infant. Clin Pediatr (Phila) 2002;41:523–524.


8. Onoyama S, Ogata R, Wada A, Saito M, Okada K, Harada T. Neonatal bacterial meningitis caused by Streptococcus gallolyticus subsp. pasteurianus. J Med Microbiol 2009;58(Pt 9): 1252–1254.


9. Cheung M, Pelot M, Nadarajah R, Kohl S. Neonate with late onset Streptococcus bovis meningitis: case report and review of the literature. Pediatr Infect Dis J 2000;19:891–893.


10. Gerber JS, Glas M, Frank G, Shah SS. Streptococcus bovis Infection in young Infants. Pediatr Infect Dis J 2006;25:1069–1073.


11. Klatte JM, Clarridge JE 3rd, Bratcher D, Selvarangan R. A longitudinal case series description of meningitis due to Streptococcus gallolyticus subsp. pasteurianus in infants. J Clin Microbiol 2012;50:57–60.



12. Cohen LF, Dunbar SA, Sirbasku DM, Clarridge JE 3rd. Streptococcus bovis infection of the central nervous system: report of two cases and review. Clin Infect Dis 1997;25:819–823.


13. Clarridge JE 3rd, Attorri SM, Zhang Q, Bartell J. 16S ribosomal DNA sequence analysis distinguishes biotypes of Streptococcus bovis: Streptococcus bovis Biotype II/2 is a separate genospecies and the predominant clinical isolate in adult males. J Clin Microbiol 2001;39:1549–1552.






 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


