Article Contents
| Korean J Pediatr > Volume 57(4); 2014 |
|
Abstract
Purpose
This study aimed to evaluate the autonomic imbalance in syncope by comparing the baseline heart rate variability (HRV) between healthy children and those with vasovagal syncope.
Methods
To characterize the autonomic profile in children experiencing vasovagal syncope, we evaluated the HRV of 23 patients aged 7-18 years and 20 healthy children. These children were divided into preadolescent (<12 years) and adolescent groups. The following time-domain indices were calculated: root mean square of the successive differences (RMSSD); standard deviation of all average R-R intervals (SDNN); and frequency domain indices including high frequency (HF), low frequency (LF), normalized high frequency, normalized low frequency, and low frequency to high frequency ratio (LF/HF).
Results
HRV values were significantly different between healthy children and those with syncope. Student t test indicated significantly higher SNDD values (60.46 ms vs. 37.42 ms, P=0.003) and RMSSD (57.90 ms vs. 26.92 ms, P=0.000) in the patient group than in the control group. In the patient group, RMSSD (80.41 ms vs. 45.89 ms, P=0.015) and normalized HF (61.18 ms vs. 43.19 ms, P=0.022) were significantly higher in adolescents, whereas normalized LF (38.81 ms vs. 56.76 ms, P=0.022) and LF/HF ratio (0.76 vs. 1.89, P=0.041) were significantly lower in adolescents. In contrast, the control group did not have significant differences in HRV values between adolescents and preadolescents.
Syncope is defined as a short period of temporally-limited and self-limited loss of consciousness due to transient diminution of blood flow to the brain. Vasovagal syncope is most common in children and adolescents, but the precise nature of such episodes remains under investigation1,2,3). To date, the main pathophysiology of vasovagal syncope is thought to be a cardioinhibitory response resulting from either increased parasympathetic activation or vasodepressor response due to inhibition of sympathetic activity, or a mixed response including both. Vasovagal syncope may be caused by autonomic cardioinhibitory and/or vasodilator response. It is likely that sensory inputs through vagal afferents, pain pathways, and central pathways affect the nucleus tractus solitaries to cause sympathoinhibition and vagal efferent activation. These responses are triggered by warm environment, prolonged standing, pain, illness, emotions, venous puncture, standing, fasting, fatigue, drug use, and menstruation. Bezold-Jarisch and carotid sinus reflexes are also thought to be involved. The Bezold-Jarisch reflex, a type of autonomic dysfunction, activates mechanoreceptors in the left ventricle when blood volume is low and stimulates sympathetic tone. Such sympathetic stimulation may result in activation of vagal afferents. The carotid sinus reflex results in increased blood pressure applied to the carotid sinus, which enhances the baroreceptor and activates vagal efferents3,4,5).
In a previous study, vasovagal syncope was defined as sympathetic-parasympathetic imbalance detected by heart rate variability (HRV), head up tilt (HUT) test and 24-hour electrocardiography (ECG) monitoring in adults. However, there were only a few HRV studies regarding vasovagal syncope in children and adolescents, and studies of baseline HRV are even more rare. There is a range of time domains (from less than 30 seconds to 24 hours) to explore cardiac autonomic tone for HRV analysis. In this study, we evaluated baseline HRV of children and adolescents with vasovagal syncope and compared the values with those of healthy controls. We hypothesized that we would detect imbalances of the autonomic nervous system in syncopal patients, and hoped to design more effective preventions.
The patient group consisted of 23 children (15 girls, 8 boys; mean age: 11.6 years) with histories of ≥1 episodes of vasovagal syncope. The patients were referred to the outpatient clinic center, Department of Pediatrics, The Catholic University of Korea, Yeouido St. Mary's Hospital between March 2010 and February 2011. In addition, the patient group was divided into preadolescents and adolescents at 12 years of age, the mean age of Korean adolescence6). All patients with symptomatic arrhythmia or other chronic underlying diseases (epilepsy, diabetes mellitus, asthma, anemia, congenital heart disease, cardiomegaly, psychological disease) or users of medication acting upon the cardiovascular system were excluded. The control group consisted of 20 children (12 girls, 8 boys; mean age 12.3 years) who never experienced syncope. The control group was recruited among patients who visited the Department of Pediatrics, The Catholic University of Korea, Bucheon St. Mary's Hospital, from February 2013 to June 2013 for treatment for the common cold. The control group was divided into preadolescents and adolescents by the age of 12 years6). It included healthy children without any chronic problems.
HRV was analyzed using an SA-3000P device (Medicore Co., Seoul, Korea). HRV recordings were obtained for 5 minutes. All study participants attached electric patches to both wrists and the left ankle and remained in relaxed sitting positions. We collected data between 9 and 11 AM to control for circadian changes of HRV. Room temperature during data collection was 23℃-25℃. All participants had fasted for at least two hours before measurements were taken. ECGs were obtained through ECG recordings using personal computers over periods of five minutes. HRV was automatically analyzed by time domain and frequency domain.
The time domain measures analyzed were the following: mean heart rate, standard deviations of all average R-R intervals (SDNN), and the square root of the mean squared differences of successive R-R intervals (RMSSD).
Frequency domain measures were calculated as the following: very low frequency component (VLF, 0.01-0.05 Hz), low frequency component (LF, 0.04-0.15 Hz), high frequency component (HF, 0.15-0.4 Hz), and low to high frequency ratio (LF/HF ratio). LF and HF were collected as normalized LF and normalized HF because of time limitations.
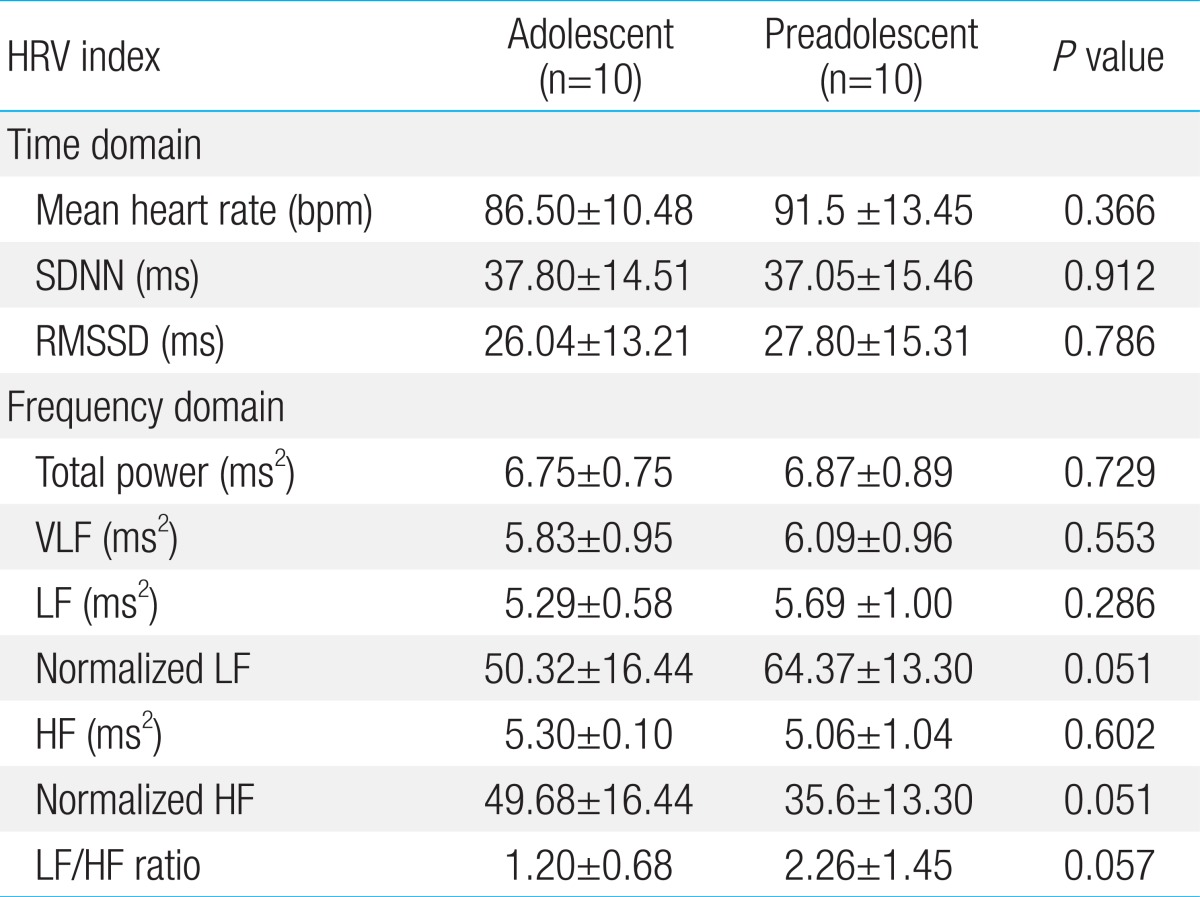
This study included 23 patients and 20 controls. There were no significant differences in age, height, or body weight between the two groups (Table 1).
The statistical analysis showed a significant increase in the median SNDD and RMSSD values in the patient group compared to the control group (P=0.003 and P=0.000).
The absolute value of normalized LF decreased in the patient group compared to the control group. Otherwise, the absolute value of normalized HF increased in the patient group compared to the control group. The LF/HF ratio decreased in the patient group compared to the control group.
HRV has become the most popular method for analyzing autonomic imbalance. Variations in heart rate are evaluated in terms of time domain measures and the frequency domain measures. The time domain measures are mean heart rate, SDNN, and RMSSD. The frequency domain is analyzed in terms of total power, VLF, HF, and LF. SDNN and LF value reflect sympathetic tone. Especially, reductions of the SDNN mean decreasing sympathetic tone by left ventricular dysfunction. RMSSD and HF reflect parasympathetic modulation of the heart. Normalized LF and normalized HF represent the relative values of LF and HF in proportion to the total power. The representations of LF and HF in normalized form mean the controlled and balanced parameter of the autonomic nervous system7). Thus, we considered normalized LF and HF to be more reliable values than LF and HF. LH/HF ratios can be used to help quantify the autonomic balance. VLF assessed from short-term recordings is a dubious measure, and not to be used1,4,7,8,9,10,11,12,13,14,15).
Comparisons of patients and controls showed that significantly increased SDNN, related to the function of the left ventricle, indicated increased stroke power in the left ventricle by activating sympathetic modulation8,10). Increasing baseline RMSSD in the patient group indicated that parasympathetic activation persisted continuously in vasovagal syncope patients. Both significantly increased SDNN and RMSSD indicated that increasing stroke volume to compensate for blood volume activates sympathetic tone, which is then followed by overstimulation of parasympathetic modulation to inhibit sympathetic increase. In the frequency domain, the results suggest relative inhibition of sympathetic tone and activation of parasympathetic tone in the patient group compared to the control group.
We also evaluated the patient group divided into adolescents and preadolescents. These results indicate that adolescents with vasovagal syncope experienced more severe activation of vagal sinus modulation than preadolescents. This result corresponds with epidemiological findings that vasovagal syncope is more frequent in adolescents than preadolescents2,5). On the other hand, adolescents and preadolescents in the control group were not significantly different in terms of HRV. This result shows that the control group regulated effectively a sympathetic-parasympathetic balance.
To confirm predominance in vagal tone of adolescent patients, we also analyzed the differences of HRV pattern by age. The time and frequency domain of HRV analysis in adolescent age show the activation of parasympathetic tone and the inhibition of sympathetic tone in adolescent patients once more.
Despite the asymptomatic period, significantly increased RMSSD and HF, decreased LF, and LF/HF ratios suggest continued hyperactive parasympathetic tone. In other words, our findings may represent cardioinhibitory response. There are some possible explanations for why this tendency is more clear in adolescents than preadolescents. Although rapid growth in adolescents requires dramatic increases in blood volume, the adolescent cardiovascular system cannot meet this challenge. Therefore, sympathetic modulation is activated to compensate deficient blood volume, and parasympathetic hyperactivation follows. This hyperactivation emerges as vasovagal syncope. As this interaction continues, parasympathetic tone usually remains hyperactive.
Unlike adults, HRV in children and adolescents with vasovagal syncope has been evaluated only infrequently. Piccirillo et al.16) detected activation of sympathetic autonomic activity and inhibition of parasympathetic activity in children with vasovagal syncope during the HUT test. Their results suggested that sympathetic tone activates during the HUT test and vagal sinus modulation increases immediately after the HUT test in vasovagal syncope patients.
Shortly before syncope, the sympathetic nervous system activates, and then parasympathetic nervous activation follows to maintain homeostasis. This result indicates the Bezold-Jarisch reflex. Longin et al.4) measured short-term HRV indices in children with syncope or presyncopal symptoms to compare with healthy children, and also found sympathetic predominance. These results were different with ours. We consider that these studies were measured just before syncope while we evaluated baseline HRV.
On the other hand, Hosaka et al.17) and Kochiadakis et al.18) reported that basal HRV indices were augmented by parasympathetic predominance in syncopal patients compared to healthy controls like our study. Guzman et al.19) found increased parasympathetic activity indicated by significant increases in RMSSD in patients with cardioinhibitory type response, and sympathetic activity predominance indicated by significant decreases in RMSSD in vasodepressor type response during HUT test. In our study, we could not be distinguished cardioinhibitory type with vasodepressor type because our patient was asymptomatic state.
The analysis of HRV patterns with vasovagal syncope remains controversial. The results of HRV analysis can be conflicting, as the pathophysiology of syncope remains unclear. More investigations are needed.
The most common medications for the prevention of vasovagal syncope are beta-blockers. These agents are thought to prevent vasovagal syncope by attenuating the sympathetically mediated increase in inotropy, making cardiac mechanoreceptor activation less likely3,20,21). These are used in children and adolescents because of their relatively small numbers of side effects20). However, despite the frequent use of beta blockers, evidence supporting the efficacy of beta blockers is extremely limited. Sheldon et al.22) found that treatment with beta-blockers did not prevent syncope recurrence. Alegria et al.23) showed that recurrent syncope was more frequent in patients treated with beta-blockers than in control patients treated conservatively. The limited evidence available on the efficacy of beta blockers is conflicting, and their role remains uncertain3,20).
We suggest some strategies for the effective prevention of vasovagal syncope based on our study. First, using anticholinergic agents to prevent vasovagal syncope may effectively inhibit vagal tone activation. There are some reports that transdermal scopolamine and propantheline bromide are effective for vasovagal syncope20,21). Although the data regarding the efficacy of anticholinergic agents are limited, such agents may be used for effective prevention if confirmed safe by continuous investigation. Second, inhibiting sympathetic overstimulation by intravascular volume expansion may be especially effective for adolescents because of their rapid growth. Fludrocortisones, the most common volume expander, is believed to exert its principal effects by increasing sodium reabsortion in the kidneys and thus increasing blood volume, limiting the hemodynamic effects of venous pooling during upright posture. However, care should be taken for use in pediatrics because adverse effects associated with fludrocortisones include hypertension, pulmonary edema, hypokalemia, hypomagnecemia. There have been some reports that fludrocortisones are effective for vasovagal syncope during short term follow up, but not effective during long-term follow-up2,3,20,21). Third, biofeedback training to increase the amplitude of respiratory sinus arrhythmia (RSA) is suggested for the prevention of vasovagal syncope. RSA is the variation in heart rate that accompanies breathing. The heart rate normally increases during inhalation and decreases during exhalation. HRV can voluntarily be controlled by respiration24). If HRV is monitored in the same way as breathing, it is possible to regulate HRV patterns, which may be an effective treatment for vasovagal syncope.
Because of significant differences of HRV between vasovagal syncope patients and controls, we recommend screening tests for children at high risk of vasovagal syncope. By screening, if children who still do not experience syncope search out, they can be prevented more effectively in advance.
Further studies are needed including larger numbers of patients to establish classifications of syncope type in terms of blood pressure. In the future, we will evaluate differences of HRV patterns in patients who follow up after starting treatment.
References
2. Benditt DG, van Dijk JG, Sutton R, Wieling W, Lin JC, Sakaguchi S, et al. Syncope. Curr Probl Cardiol 2004;29:152–229.


3. Olshansky B. Reflex syncope [Internet]. Waltham: UpToDate Inc., 2013;cited 2013 Sep 11. Available from: http://www.uptodate.com/contents/reflex-syncope.
4. Longin E, Reinhard J, von Buch C, Gerstner T, Lenz T, Konig S. Autonomic function in children and adolescents with neurocardiogenic syncope. Pediatr Cardiol 2008;29:763–770.


5. Kenny RA, Bhangu J, King-Kallimanis BL. Epidemiology of syncope/collapse in younger and older Western patient populations. Prog Cardiovasc Dis 2013;55:357–363.


6. Park MJ, Lee IS, Shin EK, Joung H, Cho SI. The timing of sexual maturation and secular trends of menarchial age in Korean adolescents. Korean J Pediatr 2006;49:610–616.

7. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 1996;93:1043–1065.


8. McCraty R, Watkins A. Autonomic assessment report: a comprehensive heart rate variability analysis-interpretation guide and instructions. Boulder Creek: Institute of HeartMath, 1996.
9. Zygmunt A, Stanczyk J. Heart rate variability in children with neurocardiogenic syncope. Clin Auton Res 2004;14:99–106.


10. Galeev AR, Igisheva LN, Kazin EM. Heart rate variability in healthy six- to sixteen-year-old children. Human Physiol 2002;28:428–432.

11. Stys A, Stys T. Current clinical applications of heart rate variability. Clin Cardiol 1998;21:719–724.


12. Lazzeri C, La Villa G, Barletta G, Franchi F. 24-hour heart rate variability in patients with vasovagal syncope. Pacing Clin Electrophysiol 2000;23(4 Pt 1): 463–468.


13. Kim JK, Kim JS, Ahn KJ, Lee SC, Gwon HC, Park SW, et al. Heart rate variability in patients with neurocardiogenic syncope or presyncope. Korean Circ J 2000;30:716–723.

14. Chun H, Kim S, Sung J, Paek D. Determinants of heart rate variability in general Korean population. Korean Circ J 2001;31:107–113.

15. Sehra R, Hubbard JE, Straka SP, Fineberg NS, Engelstein ED, Zipes DP. Autonomic changes and heart rate variability in children with neurocardiac syncope. Pediatr Cardiol 1999;20:242–247.


16. Piccirillo G, Naso C, Moise A, Lionetti M, Nocco M, Di Carlo S, et al. Heart rate and blood pressure variability in subjects with vasovagal syncope. Clin Sci (Lond) 2004;107:55–61.


17. Hosaka H, Takase B, Katsushika S, Ohsuzu F, Kurita A. Altered fractal behavior and heart rate variability in daily life in neurally mediated syncope. Biomed Pharmacother 2003;57(Suppl 1): 77S–82S.


18. Kochiadakis GE, Kanoupakis EM, Rombola AT, Igoumenidis NE, Chlouverakis GI, Vardas PE. Reproducibility of tilt table testing in patients with vasovagal syncope and its relation to variations in autonomic nervous system activity. Pacing Clin Electrophysiol 1998;21:1069–1076.


19. Guzman CE, Sanchez GM, Marquez MF, Hermosillo AG, Cardenas M. Differences in heart rate variability between cardioinhibitory and vasodepressor responses to head-up tilt table testing. Arch Med Res 1999;30:203–211.


21. Kaufmann H, Freeman R. Pharmacological treatment of reflex syncope. Clin Auton Res 2004;14(Suppl 1): 71–75.


22. Sheldon R, Rose S, Flanagan P, Koshman ML, Killam S. Effect of beta blockers on the time to first syncope recurrence in patients after a positive isoproterenol tilt table test. Am J Cardiol 1996;78:536–539.










 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


