Article Contents
| Korean J Pediatr > Volume 57(10); 2014 |
|
Abstract
Purpose
Febrile seizures are induced by fever and are the most common type of seizures in children. Although numerous studies have been performed on febrile seizures, their pathophysiology remains unclear. Recent studies have shown that cytokines may play a role in the pathogenesis of febrile seizures. The present study was conducted to identify potential links between serum interleukin-1beta (IL-1β), tumor necrosis factor-alpha (TNF-α), and febrile seizures.
Methods
Ninety-two patients with simple or complex febrile seizures (46 patients per seizure type), and 46 controls with comparable age, sex, and severity of temperature were enrolled.
Results
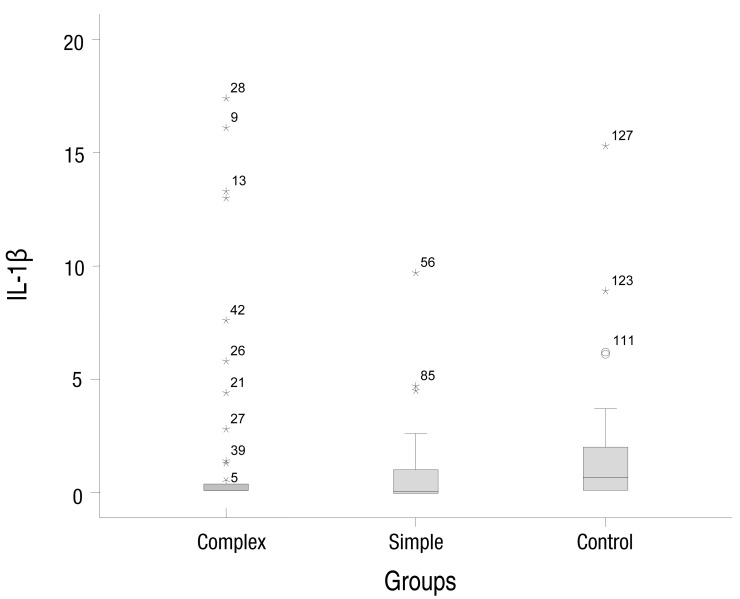
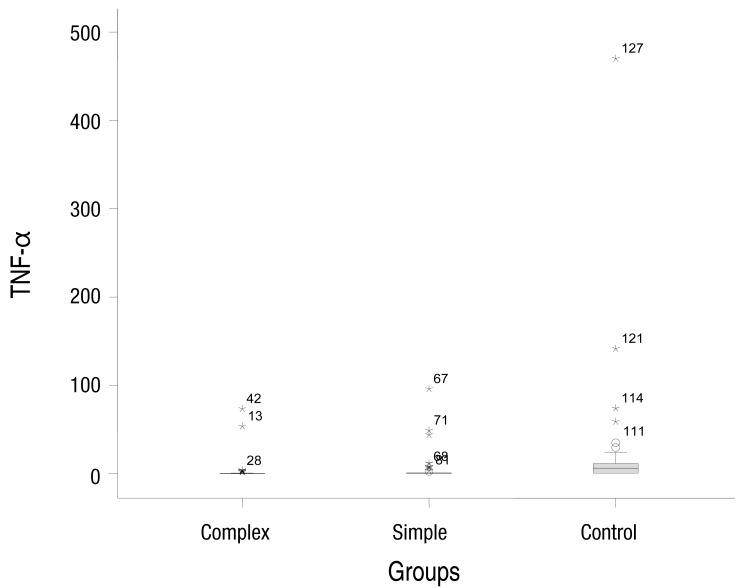
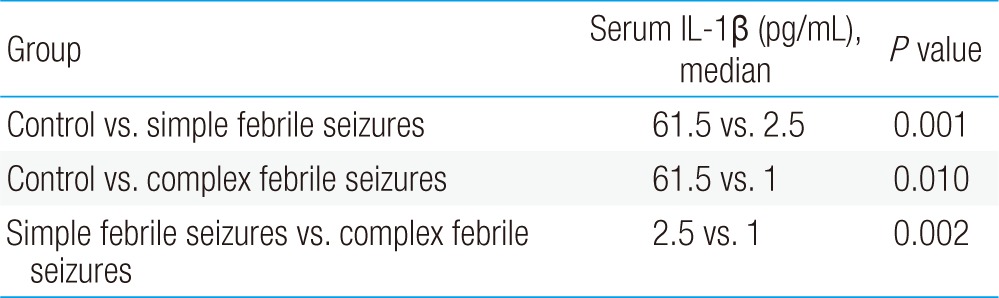
The median concentrations of serum IL-1β in the simple, complex febrile seizure, and control groups were 0.05, 0.1, and 0.67 pg/mL, respectively (P=0.001). Moreover, the median concentrations of TNF-α in the simple, complex febrile seizure, and control groups were 2.5, 1, and 61.5 pg/mL, respectively (P=0.001). Furthermore, there were significant differences between the case groups in serum IL-1β and TNF-α levels (P<0.05).
Febrile seizures are seizures induced by fever (temperature≥38℃). Febrile seizures usually occur in young children between the age of 6 months and 5 years1,2). These patients do not have any central nervous infection, electrolyte imbalance, metabolic disorders, or a history of febrile seizures1,2,3,4). Febrile seizures are classified into three types: simple, complex, and febrile status epilepticus. Simple febrile seizures are generalized, last for <15 minutes, and do not recur within 24 hours. Complex febrile seizures are more prolonged (>15 minutes), focal, and recur within 24 hours. Febrile status epilepticus seizures last for >30 minutes3,4,5,6,7,8). This type of febrile seizure occurs in 2%-9% of children9,10). While several cohort studies suggest that prognosis of febrile seizures is often good, epilepsy is observed in 5.4% of these patients2,5,11,12). Although numerous studies have been performed on febrile seizures, their exact pathophysiology remains unknown13,14). Cytokines may be one of the factors involved in the pathogenesis of febrile seizures15,16,17,18). Cytokines are hormonal mediators involved in several infectious and immunological phenomena. Interleukin-1beta (IL-1β) and tumor necrosis factor-alpha (TNF-α) are the major cytokines15,16). Tutuncuoglu et al.17) reported that increased production of IL-1β is involved in the pathogenesis of febrile seizures. In contrast, Tomoum et al.18) suggested that IL-1β do not play any roles in the pathogenesis of febrile seizures. Because of these controversial opinions, the present study was conducted to investigate the relationship between serum IL-1β and TNF-α with febrile seizures in children.
This case-control study was performed at Qazvin Children Hospital affiliated to the Qazvin University of Medical Sciences in Qazvin, Iran in 2012. Case groups (92 patients) were consecutively selected from children admitted to the hospital after simple and complex febrile seizures. Control group comprised 46 febrile children without seizures. The ages of all patients were between 6 and 60 months. Sample size was calculated to determine 95% confidence coefficient and 80% power for statistical analysis16). Consecutive sampling continued until the desired sample size was reached. Inclusion criteria for the febrile seizure groups were: (1) fever≥38℃, (2) presence of simple febrile seizure (generalized seizure lasting for <15 minutes), and (3) presence of complex febrile seizure (focal seizure lasting for >15 minutes that recurred within 24 hours)2,3). Patients with central nervous system infections, electrolyte imbalance, metabolic disorders, neurological deficits, and a history of febrile seizures were excluded. The control group included febrile children without seizures who visited the hospital clinic because of common febrile diseases such as upper respiratory tract infection. Febrile diseases (viral or bacterial) were diagnosed according to clinical findings. Body temperature (axillary) was measured using a standard method3). The ethics committee of the Research Department in the Qazvin University of Medical Sciences (project no. 284) approved the study. All parents were provided information regarding the research method in simple language. The children were included in the study after their parents agreed and signed the informed consent form. Four milliliters of blood was taken from the peripheral vessels of children in all the groups, and serum was obtained by centrifugation at 3,000 rpm for 5 minutes at 4℃. The serum was then poured into acid-washed tubes and stored in a refrigerator at -20℃ until IL-1β and TNF-α assay. In the febrile seizure groups, blood samples were collected within 5 hours after the occurrence of a seizure episode16,19). Concentrations of serum IL-1β and TNF-α were measured using enzyme-linked immunosorbent assay and commercially available kits (Cat. No. BE51011 and Cat.No.BE58351; IBL Co., Hamburg, Germany). All the samples were measured in duplicates to improve accuracy. Results were analyzed by chi-square test, analysis of variance, and nonparametric tests (Mann-Whitney U test and Kruskal-Wallis test). Cytokine levels were expressed as median and range, whereas variable values except age were expressed as mean± standard deviation. Statistical calculations were performed using SPSS ver. 15 (SPSS Inc., Chicago, IL, USA). P<0.05 was considered statistically significant.
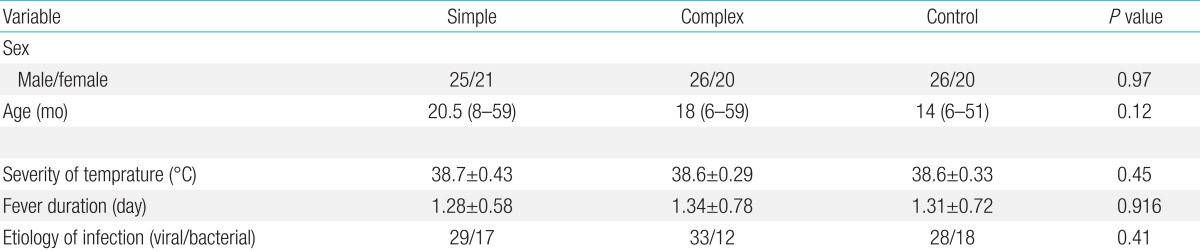
The simple febrile seizures group included 25 male and 21 female patients. These values in complex febrile seizures and control groups were 26, 20 and 26, 20 patients, respectively (P= 0.971). The minimum and maximum ages of the children in the cases and control groups were 6 and 60 months, respectively. There were no statistically significant differences between the groups with respect to age, severity of temperature, duration of fever and type of febrile disease (viral or bacterial) (P>0.05) (Table 1). The median concentrations of serum IL-1β in simple, complex febrile seizures and control groups were 0.05, 0.1, and 0.67 pg/mL, respectively (P<0.0001). Also, the median concentrations of TNF-α in simple, complex febrile seizures and control groups were 2.5, 1, and 61.5 pg/mL, respectively (P<0.0001). Serum IL-1β and TNF-α concentrations were significantly different between the febrile seizures and control groups (P<0.05) (Table 2). The simple and complex febrile seizures groups also showed significant differences regarding serum IL-1β and TNF-α levels (P<0.05) (Tables 3, 4; Figs 1, 2). No significant differences were observed between the cases and control groups with respect to the correlation between degree of fever and serum IL-1β (r=0.081, P=0.442) and TNF-α levels (r=-0.059, P=0.574).
Although numerous studies have been performed on the identification of factors causing febrile seizures in children, the actual cause of the disease has not yet been detected13,14,17,18,19,20). Few studies highlight the role of cytokines in febrile seizures16,17,18,19,20,21,22,23). Cytokines are essentially protein or glycoprotein hormones, mostly between 8 and 30 kDa that can be produced in all tissues and by most cells. Cytokines are hormonal mediators produced in body in response to defensive and growth phenomena. The role of these mediators in infectious, immunological, and inflammatory phenomena is of special interest. Cytokines include ILs, chemokines, TNFs, interferons, etc. Of these, IL-1β and TNF-α are the important cytokines15,21). One of the important role of IL-1β and TNF-α is direct and indirect modulating effects on neurons and neurotoxic neurotransmitters released during excitation or inflammation18). So, this question was raised to us; what is the role of these cytokines in febrile seizures? Previous studies have shown contradictory results regarding the role of these two cytokines in the development of febrile seizures15,16,17,18,19,20). Tutuncuoglu et al.17) reported that plasma IL-1β and cerebrospinal fluid TNF-α level in febrile seizure patients during the acute phase of the disease were significantly higher than those in controls were. However, plasma TNF-α levels and cerebrospinal fluid (CSF) IL-1β levels were not significantly different between the case and control groups. This study concluded that IL-1β is the likely factor influencing the pathogenesis of febrile seizures. Another study showed that induction of leukocytes by double-stranded RNA resulted in a large-scale production of IL-1β in febrile seizure patients as compared to that in controls; however, the levels of TNF-α did not change significantly between the two groups. This study concluded that IL-1β was a likely factor influencing the pathogenesis of febrile seizures22). Helminen et al.24) showed that stimulation of peripheral blood mononuclear cells by liposaccharide in children with febrile seizures led to an increased production of IL-1β in these children as compared to that in control. Another study showed a significant correlation between IL-1β allele 2 and febrile seizures15,25). Kanemoto et al.25) suggested that IL-1β-511T allele is a determining factor in the development of febrile seizures. A study by Dube et al.26) on an animal model showed that IL-1β plays a role in the incidence of febrile seizures by increasing N-methyl-D-aspartate function. Studies supporting the hypothesis that cytokines play an important role in the incidence of febrile seizures indicate that during infections, especially viral infections, immune cells such as macrophages, T cells, and B cells are stimulated and consequently secrete proinflammatory cytokines such as IL-1β, TNF-α, and IL-627,28,29). In addition to studies that support the role of IL-1β in the incidence of febrile seizures, there are studies that do not support the above hypothesis and suggest that no correlation exists between febrile seizures and IL-1β and TNF-α level. Lahat et al.20) reported that there was no significant difference in plasma IL-1β and cerebrospinal fluid TNF-α level between the febrile seizures children and control group. Haspolat et al.16) showed that cerebrospinal fluid IL-1β levels increased significantly in patients with febrile seizures as compared to those in controls; however, serum IL-1β and TNF-α level in the two groups were not significantly different. These results have also been confirmed by Virta et al.15) and Tomoum et al.18). Another study also showed no significant difference between children with febrile seizures and those with meningitis and encephalitis with respect to plasma concentration of IL-1β30). In the present study, serum IL-1β and TNF-α level in the simple and complex febrile seizure groups were lower than those in the control group. Moreover, no significant correlation was observed between the intensity of body temperature and the levels of IL-1β and TNF-α in the case and control groups. Lower serum levels of these cytokines in the febrile seizure groups than those in the control group indicate that production of cytokines does not increase during febrile seizures. Therefore, unlike previous studies the hypothesis that increased production of cytokines influences the incidence of febrile seizures is not convincing. Contradictory results of various studies may be due to the interference of confounding variables such as, time of sampling, severity of temperature, duration of fever, difficulty in measuring cytokines, type of infection, and sample size. Roth et al.19) showed that in guinea pigs, serum TNF-α level reached its maximum within 1 hour after injecting a bacterial endotoxin and decreased by 15%-20% after 2 hours to an extent that it was no longer measurable. The author of the above study recommended that TNF-α levels should be measured within 5 hours after the incidence of febrile seizure. Time of sampling is very important for measuring IL-1β levels. IL-1β increases within 1 hour after seizure, reaches its maximum level within 4-12 hours after seizure, and returns to its normal level after 24 hours16). Thus, IL-1β is best measured within 12 hours after the incidence of seizure. In the present study, samples were prepared within 5 hours after the incidence of seizure, a period in which actual levels of the studied cytokines can be measured. Therefore, in this study, low levels of IL-1β and TNF-α in patient with febrile seizures did not correlate with the time of sampling. Another factor that may influences cytokines level is fever. Tournieretal reported that fever increases some interleukins and decreases others31). Because, in the present study, there was no significant differences between 3 groups regarding severity of temperature (P=0.45) and duration of fever (P=0.916), thus fever cannot be a confounding variable. Another problem is measuring IL-1β level. Dinarello32) reported that measurement of IL-1β is very difficult because IL-1β binds to large proteins such as α2-macroglobulin and complements. In our study, method of measuring of interleukins was similar in all groups. It has been shown that proinflammatory cytokines increase after viral and bacterial infections21). In the present study, types of diseases (viral and bacterial) were not significantly different between the case and control groups. Most of the studied children had three types of infections: upper respiratory tract infections, viral diarrhea, and viral pneumonia. Therefore, the type of infection in the groups could not interfere with the results of this study. Sample size is another confounding factor that must be considered for evaluating the results of various studies. We calculated sample size regularly according to biostatics formula. In addition, one more explanation for low level of IL-1β and TNF-α is suppuration effect of IL-10. There are some reports that IL-10 is increased in response to lipopolysaccharide in patients with febrile convulsion8,33). Since, the etiology of disease in present study comparing the previous one is completely different, so it is not applicable in our study. Despite above discussion, still this question remains unanswered for us, whether low levels of these cytokines are related to the effect of seizure attack during infection? Responding to this question is very difficult. According to my knowledge and literature review there is no document to have satisfactory answer for this question. Donnelly et al.34) reported that IL-1β has an inhibitory effect on cell excitability and a neuroprotective role for seizure-induced hippocampal. Our study has one limitation, in that CSF IL-1β and TNF-α levels were not measured in patients with febrile seizures. The studied patients did not show any indications for undergoing lumbar puncture procedure, which is morally objectionable. As a result, unlike previous studies, our study does not support the hypothesis that increased IL-1β and TNF-α production is involved in the pathogenesis of febrile seizures.
Acknowledgment
Our thanks and best regards go to research department of Qazvin University of Medical Sciences, parents of children and Mrs. Shiva Esmaily for their cooperation.
References
2. Shinnar S. Febrile seizures. Swaiman KF, Ashwal S, Ferriero DM, editors. Pediatric neurology: principles and practice. 4th ed. Philadelphia: Mosby, 2006;:1079–1086.
3. Mikati MA. Febrile seizures. Kliegman RM, Stanton BF, Geme JW, Schor NF, Behrman RE, editors. Nelson textbook of pediatrics. 19th. Philadelphia: Saunders, 2011;:2017–2019.
4. Graves RC, Oehler K, Tingle LE. Febrile seizures: risks, evaluation, and prognosis. Am Fam Physician 2012;85:149–153.

9. Baumann RJ, Duffner PK. Treatment of children with simple febrile seizures: the AAP practice parameter. American Academy of Pediatrics. Pediatr Neurol 2000;23:11–17.


10. Nakayama J, Arinami T. Molecular genetics of febrile seizures. Epilepsy Res 2006;70(Suppl 1): S190–S198.

11. Birca A, Guy N, Fortier I, Cossette P, Lortie A, Carmant L. Genetic influence on the clinical characteristics and outcome of febrile seizures: a retrospective study. Eur J Paediatr Neurol 2005;9:339–345.


12. Vestergaard M, Pedersen CB, Sidenius P, Olsen J, Christensen J. The long-term risk of epilepsy after febrile seizures in susceptible subgroups. Am J Epidemiol 2007;165:911–918.


13. Jan MM, Girvin JP. Febrile seizures. Update and controversies. Neurosciences (Riyadh) 2004;9:235–242.

15. Virta M, Hurme M, Helminen M. Increased plasma levels of proand anti-inflammatory cytokines in patients with febrile seizures. Epilepsia 2002;43:920–923.


16. Haspolat S, Mihci E, Coskun M, Gumuslu S, Ozben T, Yegin O. Interleukin-1beta, tumor necrosis factor-alpha, and nitrite levels in febrile seizures. J Child Neurol 2002;17:749–751.


17. Tutuncuoglu S, Kutukculer N, Kepe L, Coker C, Berdeli A, Tekgul H. Proinflammatory cytokines, prostaglandins and zinc in febrile convulsions. Pediatr Int 2001;43:235–239.


18. Tomoum HY, Badawy NM, Mostafa AA, Harb MY. Plasma interleukin-1beta levels in children with febrile seizures. J Child Neurol 2007;22:689–692.


19. Roth J, Conn CA, Kluger MJ, Zeisberger E. Kinetics of systemic and intrahypothalamic IL-6 and tumor necrosis factor during endotoxin fever in guinea pigs. Am J Physiol 1993;265(3 Pt 2): R653–R658.


20. Lahat E, Livne M, Barr J, Katz Y. Interleukin-1beta levels in serum and cerebrospinal fluid of children with febrile seizures. Pediatr Neurol 1997;17:34–36.


22. Ichiyama T, Suenaga N, Kajimoto M, Tohyama J, Isumi H, Kubota M, et al. Serum and CSF levels of cytokines in acute encephalopathy following prolonged febrile seizures. Brain Dev 2008;30:47–52.


23. Matsuo M, Sasaki K, Ichimaru T, Nakazato S, Hamasaki Y. Increased IL-1beta production from dsRNA-stimulated leukocytes in febrile seizures. Pediatr Neurol 2006;35:102–106.


24. Helminen M, Vesikari T. Increased interleukin-1 (IL-1) production from LPS-stimulated peripheral blood monocytes in children with febrile convulsions. Acta Paediatr Scand 1990;79:810–816.


25. Kanemoto K, Kawasaki J, Yuasa S, Kumaki T, Tomohiro O, Kaji R, et al. Increased frequency of interleukin-1beta-511T allele in patients with temporal lobe epilepsy, hippocampal sclerosis, and prolonged febrile convulsion. Epilepsia 2003;44:796–799.


26. Dube C, Vezzani A, Behrens M, Bartfai T, Baram TZ. Interleukin-1beta contributes to the generation of experimental febrile seizures. Ann Neurol 2005;57:152–155.



27. Yuhas Y, Shulman L, Weizman A, Kaminsky E, Vanichkin A, Ashkenazi S. Involvement of tumor necrosis factor alpha and interleukin-1beta in enhancement of pentylenetetrazole-induced seizures caused by Shigella dysenteriae. Infect Immun 1999;67:1455–1460.



28. Watkins LR, Goehler LE, Relton J, Brewer MT, Maier SF. Mechanisms of tumor necrosis factor-alpha (TNF-alpha) hyperalgesia. Brain Res 1995;692:244–250.


29. Muñoz-Fernandez MA, Fresno M. The role of tumour necrosis factor, interleukin 6, interferon-gamma and inducible nitric oxide synthase in the development and pathology of the nervous system. Prog Neurobiol 1998;56:307–340.


30. Ichiyama T, Nishikawa M, Yoshitomi T, Hayashi T, Furukawa S. Tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-6 in cerebrospinal fluid from children with prolonged febrile seizures. Comparison with acute encephalitis/encephalopathy. Neurology 1998;50:407–411.


31. Tournier JN, Hellmann AQ, Lesca G, Jouan A, Drouet E, Mathieu J. Fever-like thermal conditions regulate the activation of maturing dendritic cells. J Leukoc Biol 2003;73:493–501.







 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


