Article Contents
| Korean J Pediatr > Volume 57(11); 2014 |
|
Abstract
Purpose
The aims of this study were to document our single-center experience with pediatric acute fulminant myocarditis (AFM) and to investigate its clinical features and short-term outcomes.
Methods
We performed a retrospective chart review of all children <18 years old who were diagnosed with AFM between October 2008 and February 2013. Data about patient demographics, initial symptoms, investigation results, management, and outcomes between survivors and nonsurvivors were collected.
Results
Seventeen of 21 patients (80.9%) with myocarditis were diagnosed with AFM. Eleven patients (64.7%) survived to discharge, and 6 (35.3%) died. Electrocardiography on admission revealed dysrhythmia in 10 patients (58.8%); of these, all 7 patients with a complete atrioventricular block survived. Fractional shortening upon admission was significantly different between the survivors (16%) and nonsurvivors (8.5%) (P=0.01). Of the serial biochemical markers, only the initial brain natriuretic peptide (P=0.03) and peak blood urea nitrogen levels (P=0.02) were significantly different. Of 17 patients, 4 (23.5%) required medical treatment only. Extracorporeal membrane oxygenation (ECMO) was performed in 13 patients (76.5%); the survival rate in these patients was 53.8%. ECMO support was initiated >24 hours after admission in 4 of the 13 patients (30.7%), and 3 of those 4 patients (75%) died.
Acute fulminant myocarditis (AFM) is a rare and distinct clinical entity whose exact incidence is unknown; it constitutes approximately 10%-38% of all cases of acute myocarditis1,2,3). While the clinical presentation of myocarditis can be acute or chronic in adults, the majority of children with myocarditis present with acute or fulminant disease4).
Myocarditis results from inflammation of the heart muscle associated with apoptotic degeneration, with or without necrosis of adjacent myocytes5). Myocellular damage results in myocardial dysfunction leading to heart failure, and AFM is characterized by sudden onset of aggressive conditions such as, fatal arrhythmias, atrioventricular block (AV), severe congestive heart failure or cardiogenic shock following a flu-like illness2,6). Patients with AFM typically have a short viral prodrome with a distinct onset of symptoms (≤14 days) and rapid deterioration requiring hemodynamic support with inotropes and mechanical ventilation6). Mechanical circulatory assistance such as by extracorporeal membrane oxygenation (ECMO) or ventricular assist device (VAD) can be required7,8,9,10,11).
The mortality rates for infants and children with myocarditis may be as high as 75% and 25%12) respectively, and postmortem examinations in routine clinical settings suggest that myocarditis is an important cause of acute cardiac death in both pediatric and adult populations13).
This study aimed at documenting our single-center experience with AFM in pediatric patients and investigating the clinical features and short-term outcome of these children.
We performed a retrospective chart review of all children under 18 years old who were diagnosed with AFM, at the Pusan National University Children's Hospital, between October 2008 and February 2013 (n=17). Seventeen patients met the inclusion criteria for AFM. AFM was diagnosed on the basis of clinical features at presentation: (1) recent history of viral prodrome with fever lasting χ2 weeks; (2) development of acute and severe heart failure after viral illness, such as heart failure with symptoms including dyspnea and orthopnea, low cardiac output, which includes abdominal discomfort, seizure, and syncope; (3) evidence of myocardial damage including significant changes in electrocardiography (EKG), elevation in cardiac markers (creatine kinase-myoglobin [CK-MB), troponin I [TnI], or myoglobin), or cardiac dysfunction on echocardiogram (ejection fraction [EF]<40% and fractional shortening [FS]<25%, as measure using M-mode in the parasternal long-axis view via the leading-edge to leading-edge method); and (4) no previous history of heart disease.
Patient demographics, initial symptoms, duration of symptoms before admission, laboratory details, electrocardiographic and chest radiographic data, and hemodynamics measured by echocardiogram were collected. Values at admission and peak values of laboratory markers were collected, including CK-MB, TnI, myoglobin, brain natriuretic peptide (BNP), aspartate aminotransferase, alanine aminotransferase, blood urea nitrogen (BUN), and creatinine.
Viral studies to identify the infectious cause of myocarditis were recorded when available. Viral pathogens were detected via multiplex polymerase chain reaction (PCR) of specimens collected by nasopharyngeal swabs, and enteroviral specimens were obtained via PCR of a specimen collected from patients' stool.
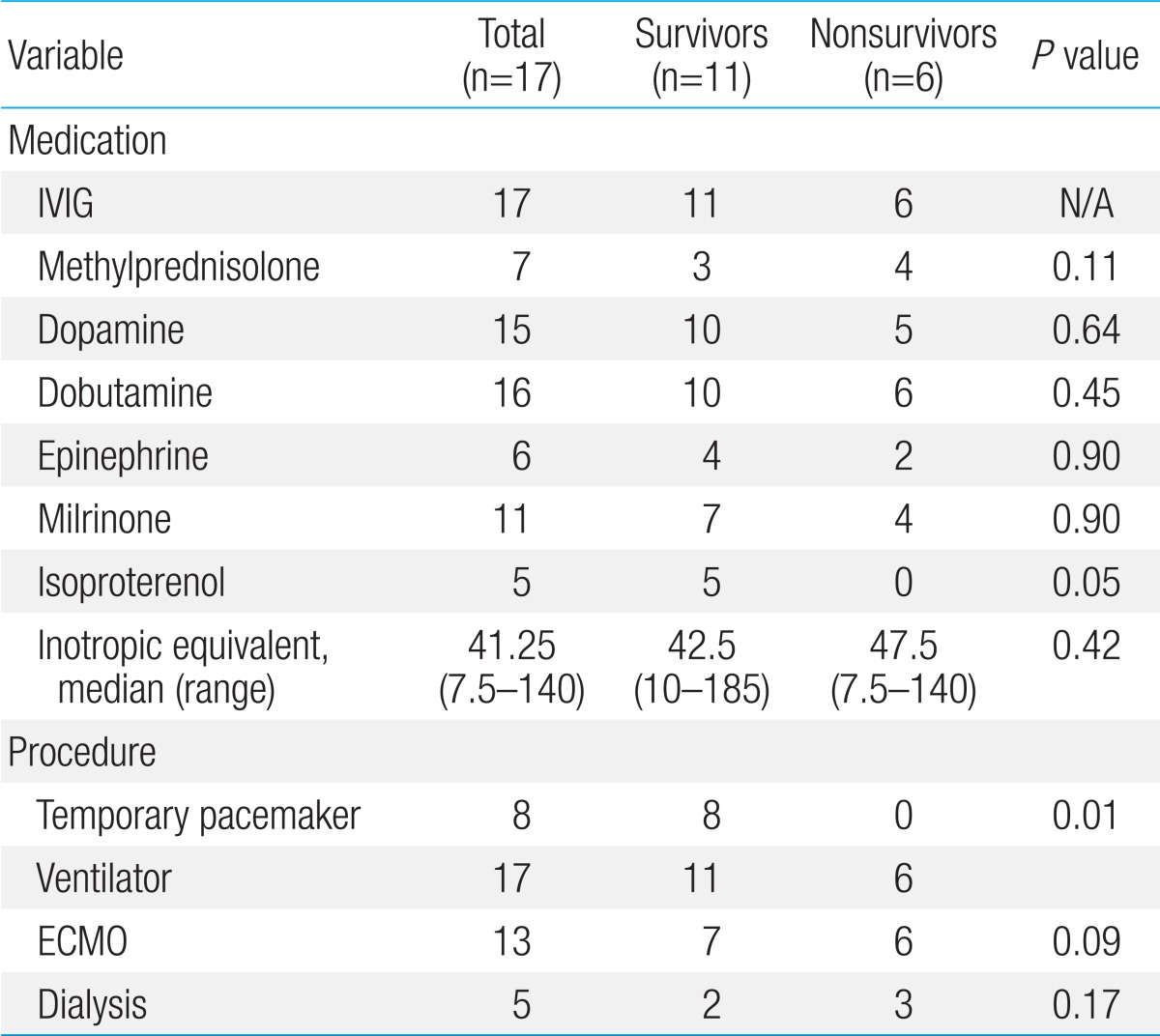
Data were also collected regarding treatments, including immunomodulatory therapy, inotropic equivalents6,9) (IE, µg/kg/min=dopamine+dobutamine+[15×milrinone]+[100×epinephrine]+[100×norepinephrine]+[100×isoproterenol]), ventilator support, temporary pacemaker insertion, dialysis and ECMO.
The patients were divided into two groups based on survival at their hospital discharge.
The following were indications for the ECMO therapy, (1) hypotension (<60 mmHg for children and <50 mmHg for infants) despite an IE >40 µg/kg/min, (2) cardiac arrest requiring continuous cardiopulmonary resuscitation (CPR), (3) cardiac and pulmonary failure refractory to medical therapy, and (4) uncontrolled arrhythmia including ventricular tachycardia (VT) and ventricular fibrillation8,9,14).
The patients were maintained on a continuous heparin infusion to achieve an activated clotting time between 180 and 200 seconds. The goal for the activated clotting time was adjusted if there were issues with bleeding or coagulation. To maintain a hemoglobin level of ≥10 g/dL and a platelet count of ≥100,000/dL, patients received a transfusion during the ECMO support9,10).
The cannulation site was determined by patient status. The majority of the patients underwent percutaneous cannulation, through either the neck or femoral vessels. Central cannulation via a median sternotomy was used only if left-side decompression was required.
Weaning from ECMO was evaluated through a multidisciplinary approach on a daily basis. A transthoracic echocardiogram was performed every 24 hours to 48 hours at a reduced ECMO flow rate. Decannulation was performed after satisfactory cardiac function and oxygen delivery were achieved15).
Data were analyzed using SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA). The values are presented as the median (range). All demographic data, clinical data and laboratory findings were compared between the two groups (survivors and nonsurvivors) using the Wilcoxon signed rank test and Mann-Whitney U test for other variables. To compare the proportions of patients, Fisher exact test was performed. All statistical tests were two-sided and significance was defined as P<0.05.
Seventeen out of 21 patients (80.9%) with myocarditis were diagnosed with AFM. The patient characteristics are shown in Table 1. Of the 17 patients who met the clinical criteria for AFM, 14 (82.4%) were girls. The median age was 4.33 years (range, 0.17-14 years), and the median body weight was 14.5 kg (range, 4-45 kg).
Out of 17 patients, 11 (64.7%) survived, and 6 (35.3%) died. There were 9 girls (81.8%) in the survivor group and 5 girls (83.3%) (P=0.94) in the nonsurvivor groups. For survivors and nonsurvivors, the median age was 5.58 years (range, 0.17-13 years) and 4.6 years (range, 0.25-14 years) (P=0.33), and the median body weight was 19.74 kg (range, 4.1-40 kg) and 17.5 kg (range, 8-45 kg) (P=0.35), respectively. The differences in patients' demographics were not statistically significant between the survivors and nonsurvivors.
No significant differences were observed in the initial symptoms between the survivors and nonsurvivors (Table 1). The median time from the first presentation to a medical provider because of symptoms until hospital admission was 3 days (range, 2-7 days) in survivors and 7 days (range, 1-10 days) in the nonsurvivors (P=0.05), which bordered on significance, with a longer time observed in patients who did not survive.
Cardiomegaly (cardiothoracic ratio>0.5) was detected on chest radiography in all 17 patients (100%). The EKG examination on admission revealed abnormal rhythms in 10 patients (58.8%); all 7 patients with a complete AV block survived (P=0.01).
EF was obtained in all 17 patients with a median value of 30% (range, 6%-52%). FS was also measured in all patients with a median value of 16% (range, 3%-25%). The qualitative degree of mitral regurgitation (MR) was assessed in 17 patients, with moderate MR noted in 12 (70.6%) and trivial to mild MR noted in 5 (29.4%). The median FS detected using an echocardiographic evaluation upon arrival to our Emergency Department was significantly different (P=0.04) between the survivors (16%) and nonsurvivors (8.5%).
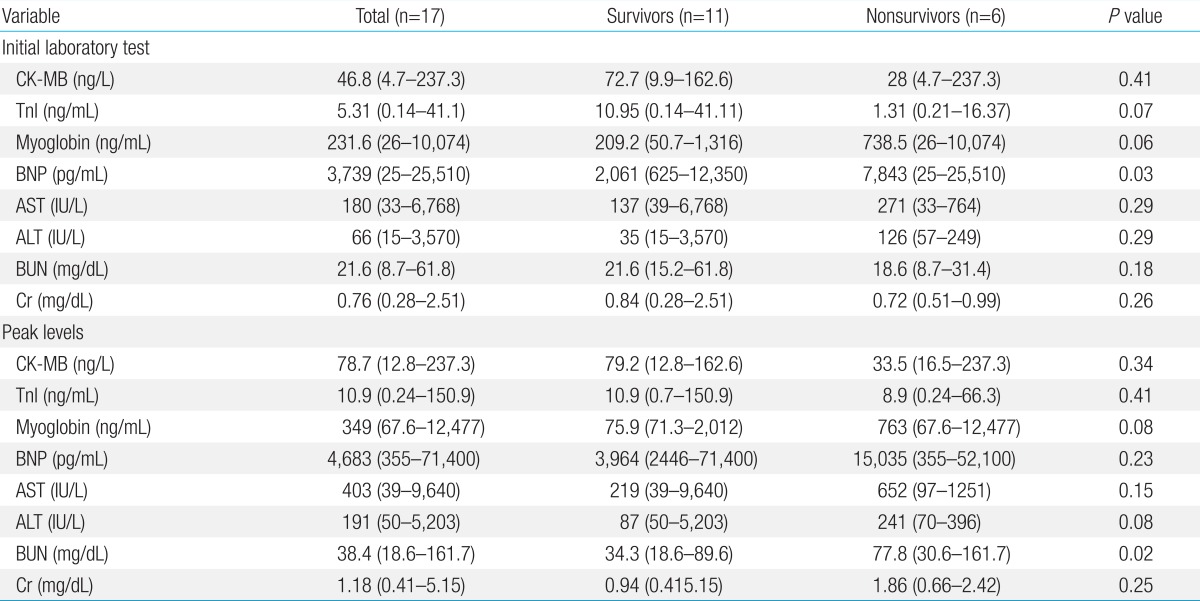
The results of the initial laboratory tests conducted upon admission and the peak levels of the same variables are provided in Table 2. Of the 4 cardiac enzymes that were measured, only initial BNP level was significantly higher in the nonsurvivors (7,843 pg/mL; range, 25-25,510 pg/mL) than in the survivors (2,061 pg/mL; range, 625-12,350 pg/mL) (P=0.03). Of the renal indicators, only the peak BUN levels were significantly higher in the nonsurvivors (77.8 mg/dL; range, 30.6-161.7 mg/dL) than in the survivors (34.3 mg/dL; range, 18.6-89.6 mg/dL) (P=0.02).
Viral studies were available for 13 of 17 patients (76.5%), and viral pathogens were detected in only 3 of those 13 patients (23.1%): influenza B virus, parainfluenza virus, and adenovirus.
Inotropic agents were required due to hemodynamic instabilities in most patients (Table 3). All 17 patients received intravenous immunoglobulin (2 g/kg) as soon as the diagnosis of myocarditis was considered. All of the patients required ventilator support. A temporary pacemaker was inserted for complete AV block in 8 patients (47.1%), all of whom were survivors (P=0.01). Out of 8 patients, 7 (87.5%) showed complete AV block on initial EKG (Table 1). One patient initially showed VT on admission EKG. Although he underwent direct current cardioversion, he did not recover to normal sinus rhythm, and showed complete AV block; therefore, a temporary pacemaker was inserted. Dialysis for renal failure was required in 5 patients (29.4%), and the distribution between the survivors and nonsurvivors was not significant.
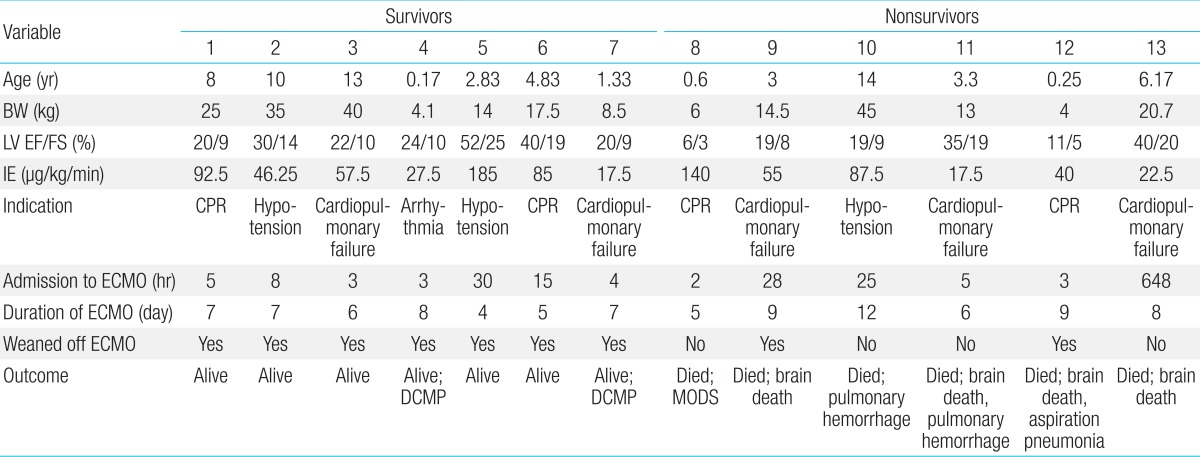
Mechanical support such as ECMO was used in 13 patients (76.5%), whom met the indications for ECMO (CPR, hypotension, cardiopulmonary failure, and refractory arrhythmia) (Table 4). Four patients (23.5%) were removed from ECMO support, because of relatively stable hemodynamic parameters, and a good response to medical treatment. The median age was 5.19 years (range, 0.17-14 years), median body weight was 19.02 kg (range, 4.0-45 kg), and the median duration of symptoms before admission was 5.08 days (range, 1-10 days). The median left ventricular (LV) EF was 26% (range, 6%-52%) and the median FS was 12.3% (range, 3%-25%).
The average use of IE before ECMO cannulation was 67.2 µg/kg/min (range, 17.5-180 µg/kg/min). Twelve patients (92.3%) were cannulated percutaneously through the internal jugular vein and carotid artery (83.3%), internal jugular vein and femoral artery (8.3%) or femoral vein and artery (8.3%). One patient (0.15%) underwent open chest cannulation to the right atrium and ascending aorta. One patient required decompression procedure for left heart distension and pulmonary edema with left atrial drainage.
The median time to ECMO cannulation after admission to our center was 5 hours (2-648 hours). Initiation of the ECMO support occurred >24 hours after admission for 4 of the 13 patients (30.7%). One patient received ECMO support on day of 27 of the hospital admission; initially, the patient responded to inotropic treatment, but experienced worsening of the cardiopulmonary failure. The median duration of the ECMO support was 7 days (range, 4-11 days).
Complications of ECMO included bleeding at the cannulation site in 8 patients (61.5%), acute renal failure in 6 patients (46.2%), brain death in 5 patients (38.5%), pulmonary hemorrhage in 2 patients (15.4%), infection at the cannulation site in 2 patients (15.4%), Horner syndrome in 1 patient (7.7%), and vocal cord paralysis in 1 patient (7.7%).
Overall, 11 patients (64.7%) survived to discharge, and 6 (35.3%) died (Fig. 1). Of the patients who required only medical treatment (4/17, 23.5%), all survived. Decannulation occurred in 9 (69.2%) of the 13 patients who required ECMO; of these, 2 (22.2%) died from brain death and aspiration pneumonia. The decannulation failed in 4 of 13 patients (44.4%), and these patients died from multiorgan dysfunction, pulmonary hemorrhage, or brain death.
The AV block persisted in 1 patient who had survived, and a permanent pacemaker was inserted. Two patients developed dilated cardiomyopathy (DCMP), and they were followed up with seri al echocardiography on every subsequent visit to the hospital (Fig. 2). The FS was lower in the early treatment phase (within 48 hours of management); however, we observed after serial follow up that, at discharge, 13 patients had improved LV FS. At the last follow-up, 2 of these patients had decreased FS and then developed DCMP.
AFM requires inotropic support or mechanical cardiopulmonary assist devices such as ECMO or VAD16,17) because of severe hemodynamic deterioration, cardiogenic shock, severe ventricular dysfunction, and refractory arrhythmia.
We encountered 17 children with AFM in the previous 5 years. The diagnosis of AFM was largely based on clinical manifestations, some biochemical markers, EKG, and echocardiographic characteristics. We did not perform endomyocardial biopsy in any of the children owing to risks associated with the procedure, particularly in children with dilated hearts17,18). Moreover, biopsy results take time; therefore, we decided to manage children with suggestive clinical features without waiting for biopsy results1,2).
Because of the nonspecific signs and symptoms in AFM, 71% children are misdiagnosed with either sepsis or pneumonia12,19). In our study most patients initially had nonspecific symptoms such as fever, respiratory symptoms (cough, rhinorrhea, and dyspnea), and gastrointestinal symptoms (nausea, vomiting, and abdominal pain). It is suspected that these symptoms at initial presentation delay the diagnosis of myocarditis, and this delay results in delayed hospitalization, thus increasing mortality rates. Earlier hospital admission may aid recovery from AFM, as indicated by the favorable clinical outcomes obtained in the present study.
In our study, patients who had complete AV block on the initial EKG had higher survival rates. In contrast, Miyake et al.20) reported poorer outcomes in patients with arrhythmias, especially in the half of those who were hospitalized. However, we presume that the good outcomes of the patients in our study emphasize the importance of early detection of AFM, as inidicated by the complete AV block on the initial EKG. Therefore, patients showing dysrhythmia on the initial EKG could undergo early cardiac evaluation and could receive early treatments such as insertion of a temporary pacemaker.
Despite low statistical significance, we recommend that myocarditis be considered in children with varying presenting symptoms that are associated with cardiomegaly on chest radiography or abnormal findings on EKG (Complete AV block; VT/VF).
Al-Biltagi et al.21) reported that low LV EF at admission was significantly associated with poor immediate outcomes. In this study, both median LV EF and FS at admission were lower in the patients who did not survive than that in those who survived, although this difference was significant only for FS. Overall, although the numbers are too small to compare the survival rates, the degree of LV dysfunction at admission was found to be significantly associated with patient outcomes. We observed after the serial follow-up, myocardial recovery started early after hospital admission, and by the time of discharge, 13 patients had improved LV FS. Previous data by Felker et al.22) describing myocardial recovery reported significant improvement in %FS in the AFM, similar to the results of our study.
Higher levels of serial biochemical markers were observed in patients who did not survive, and these differences were significant for BNP at admission and the peak BUN level. Increased levels of the renal function indicators suggest renal failure, which is associated with increased mortality23). By contrast, Chen et al.24) reported that peak troponin levels indicated more severe myocardial damage. In our study the peak BNP level was significantly higher than the peak troponin level. Therefore, although the initial cardiac enzyme level had low predictive value, we could use these peak cardiac enzyme levels to predict the potentiality of myocardial recovery.
The management of AFM depends on its severity at initial presentation and may vary from use of ECMO to that of VAD. In recent reports of ECMO support in children with acute myocarditis, the survival rate was 59.6%-67%9,10,11). Although the survival rate in children with AFM was similar (64.7%), the patients that received ECMO support generally showed a relatively low survival rate (53.8%). We assumed the factors related to low survival rate were the lack of experience with these mechanical supports (especially ECMO). However, as we encountered more patients with AFM, the death rates declined considerably. Delayed initiation of ECMO support (>24 hours after admission) also appears to be associated with poor prognosis, with death occurring in 75% of the patients with delayed ECMO initiation in the present study. Furthermore, the Extracorporeal Life Support Organization registry data show that ECMO support that lasts >12.5 days increases mortality rates in cardiac patients25).
Of the patients who received ECMO, 5 (83.3%) had neurologic complications (cerebral infarction or hemorrhage) that appeared to be related to ECMO. Therefore, once a patient has been transitioned to ECMO support, the proper indications for ECMO should be emphasized to minimize complications related to such procedures.
Our study had certain limitations. First, owing to data collection at a single center, the findings may not be representative of the general population. Second, this study collected data via a retrospective chart review. Therefore, the data are dependent on historical records. Third, histopathological confirmation of AFM is lacking.
AFM with hemodynamic instability is associated with high rates of mortality, even with appropriate medical treatment in some patients. Based on our experience, AFM outcomes may be associated with the duration from symptom onset to hospital admission, LV FS at admission, the time to initiate ECMO support, biomarkers for renal function, and ECMO-related neurologic complications.
To conclude, the duration of symptoms before hospital admission, arrhythmia on initial EKG, and echocardiographic features were similar to those in previous studies. However, we would like to emphasize the importance of early recognition of the disease, because an early decision to start mechanical circulatory support improved patient outcomes. Moreover, ECMO is a highly effective form of hemodynamic support for patients with fulminant myocarditis.
References
1. Amabile N, Fraisse A, Bouvenot J, Chetaille P, Ovaert C. Outcome of acute fulminant myocarditis in children. Heart 2006;92:1269–1273.



2. Sankar J, Khalil S, Jeeva Sankar M, Kumar D, Dubey N. Short-term outcomes of acute fulminant myocarditis in children. Pediatr Cardiol 2011;32:885–890.


3. McCarthy RE 3rd, Boehmer JP, Hruban RH, Hutchins GM, Kasper EK, Hare JM, et al. Long-term outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis. N Engl J Med 2000;342:690–695.


4. Tschope C, Bock CT, Kasner M, Noutsias M, Westermann D, Schwimmbeck PL, et al. High prevalence of cardiac parvovirus B19 infection in patients with isolated left ventricular diastolic dysfunction. Circulation 2005;111:879–886.


5. Esfandiarei M, McManus BM. Molecular biology and pathogenesis of viral myocarditis. Annu Rev Pathol 2008;3:127–155.


6. Teele SA, Allan CK, Laussen PC, Newburger JW, Gauvreau K, Thiagarajan RR. Management and outcomes in pediatric patients presenting with acute fulminant myocarditis. J Pediatr 2011;158:638–643.e1.


7. Brown KL, Ichord R, Marino BS, Thiagarajan RR. Outcomes following extracorporeal membrane oxygenation in children with cardiac disease. Pediatr Crit Care Med 2013;14(5 Suppl 1): S73–S83.


8. del Nido PJ. Extracorporeal membrane oxygenation for cardiac support in children. Ann Thorac Surg 1996;61:336–339.


9. Hsu KH, Chi NH, Yu HY, Wang CH, Huang SC, Wang SS, et al. Extracorporeal membranous oxygenation support for acute fulminant myocarditis: analysis of a single center's experience. Eur J Cardiothorac Surg 2011;40:682–688.

10. Rajagopal SK, Almond CS, Laussen PC, Rycus PT, Wypij D, Thiagarajan RR. Extracorporeal membrane oxygenation for the support of infants, children, and young adults with acute myocarditis: a review of the Extracorporeal Life Support Organization registry. Crit Care Med 2010;38:382–387.



11. Wilmot I, Morales DL, Price JF, Rossano JW, Kim JJ, Decker JA, et al. Effectiveness of mechanical circulatory support in children with acute fulminant and persistent myocarditis. J Card Fail 2011;17:487–494.


12. Freedman SB, Haladyn JK, Floh A, Kirsh JA, Taylor G, Thull-Freedman J. Pediatric myocarditis: emergency department clinical findings and diagnostic evaluation. Pediatrics 2007;120:1278–1285.


13. Doolan A, Langlois N, Semsarian C. Causes of sudden cardiac death in young Australians. Med J Aust 2004;180:110–112.


14. Fleming GM, Gurney JG, Donohue JE, Remenapp RT, Annich GM. Mechanical component failures in 28,171 neonatal and pediatric extracorporeal membrane oxygenation courses from 1987 to 2006. Pediatr Crit Care Med 2009;10:439–444.


15. Thourani VH, Kirshbom PM, Kanter KR, Simsic J, Kogon BE, Wagoner S, et al. Venoarterial extracorporeal membrane oxygenation (VA-ECMO) in pediatric cardiac support. Ann Thorac Surg 2006;82:138–144.


16. Saji T, Matsuura H, Hasegawa K, Nishikawa T, Yamamoto E, Ohki H, et al. Comparison of the clinical presentation, treatment, and outcome of fulminant and acute myocarditis in children. Circ J 2012;76:1222–1228.


17. Lieberman EB, Hutchins GM, Herskowitz A, Rose NR, Baughman KL. Clinicopathologic description of myocarditis. J Am Coll Cardiol 1991;18:1617–1626.


18. Baraldi-Junkins C, Levin HR, Kasper EK, Rayburn BK, Herskowitz A, Baughman KL. Complications of endomyocardial biopsy in heart transplant patients. J Heart Lung Transplant 1993;12(1 Pt 1): 63–67.

19. Press S, Lipkind RS. Acute myocarditis in infants Initial presentation. Clin Pediatr (Phila) 1990;29:73–76.


20. Miyake CY, Teele SA, Chen L, Motonaga KS, Dubin AM, Balasubramanian S, et al. In-hospital arrhythmia development and outcomes in pediatric patients with acute myocarditis. Am J Cardiol 2014;113:535–540.


21. Al-Biltagi M, Issa M, Hagar HA, Abdel-Hafez M, Aziz NA. Circulating cardiac troponins levels and cardiac dysfunction in children with acute and fulminant viral myocarditis. Acta Paediatr 2010;99:1510–1516.


22. Felker GM, Boehmer JP, Hruban RH, Hutchins GM, Kasper EK, Baughman KL, et al. Echocardiographic findings in fulminant and acute myocarditis. J Am Coll Cardiol 2000;36:227–232.


23. Duncan BW, Bohn DJ, Atz AM, French JW, Laussen PC, Wessel DL. Mechanical circulatory support for the treatment of children with acute fulminant myocarditis. J Thorac Cardiovasc Surg 2001;122:440–448.


Fig. 1
Outcomes of pediatric patients with acute fulminant myocarditis. ECMO, extracorporeal membrane oxygenation; DCMP, dilated cardiomyopathy.
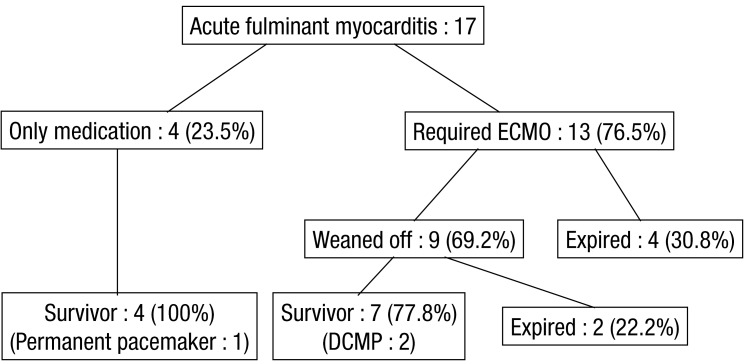
Fig. 2
Serial changes in fractional shortening of pediatric patients with acute fulminant myocarditis (n=17). ECMO, extracorporeal membrane oxygenation; F/U, follow-up.
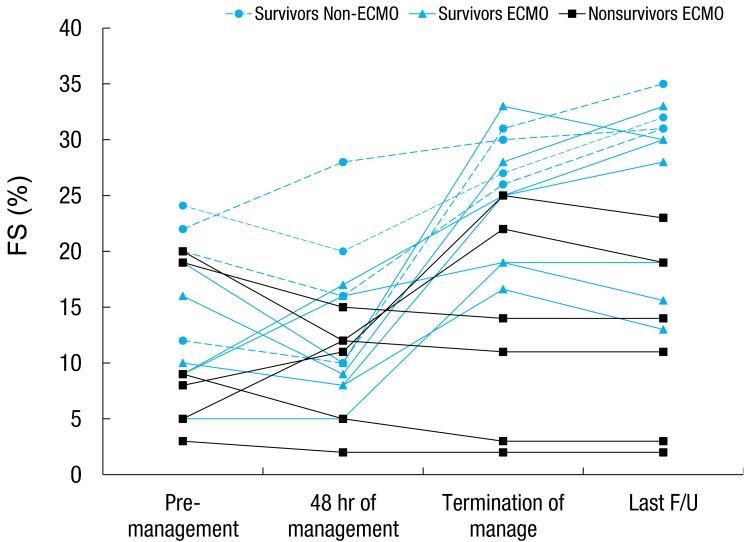
Table 1
Characteristics of pediatric patients with acute fulminant myocarditis
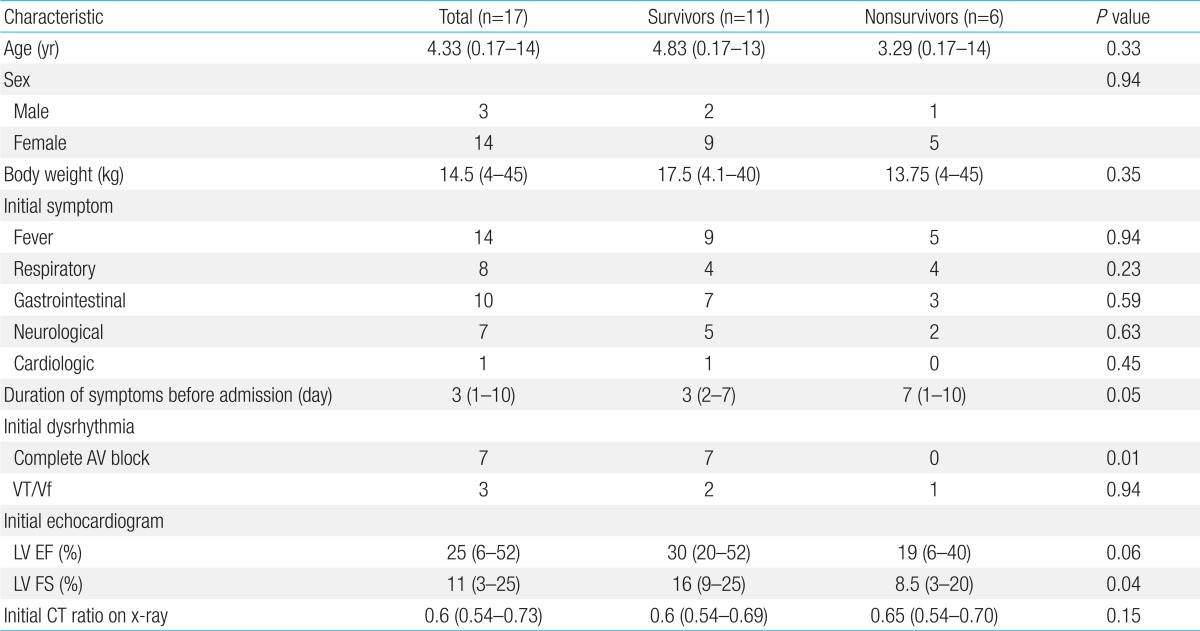
Values are presented as median (range) or number.
Respiratory symptoms included cough, rhinorrhea, and dyspnea; gastrointestinal symptoms included nausea, vomiting, and abdominal pain; neurologic symptoms included seizure and dizziness; and cardiologic symptoms included palpitations and chest pain.
BW, body weight; AV, atrioventricular; VT, ventricular tachycardia; Vf, ventricular fibrillation; LV, left ventricular; EF, ejection fraction; FS, fractional shortening; CT ratio, cardiothoracic ratio.



 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


