Article Contents
| Korean J Pediatr > Volume 57(11); 2014 |
|
Abstract
Purpose
Chronic upper airway obstruction causes hypoxemic pulmonary vasoconstriction, which may lead to right ventricle (RV) dysfunction. Adenotonsillar hypertrophy (ATH) is the most common cause of upper airway obstruction in children. Therefore, we aimed to evaluate RV function in children with ATH.
Methods
Twenty-one children (male/female, 15/6; mean age, 92.3┬Ī┬Š39.0 months; age range, 4-15 years) with ATH and 21 healthy age- and gender-matched controls were included in this study. Tricuspid annular plane systolic excursion and RV myocardial performance index were measured by transthoracic echocardiography. Further, the plasma level of N-terminal of probrain natriuretic peptide (NT-proBNP), an indicator of RV function, was determined.
Results
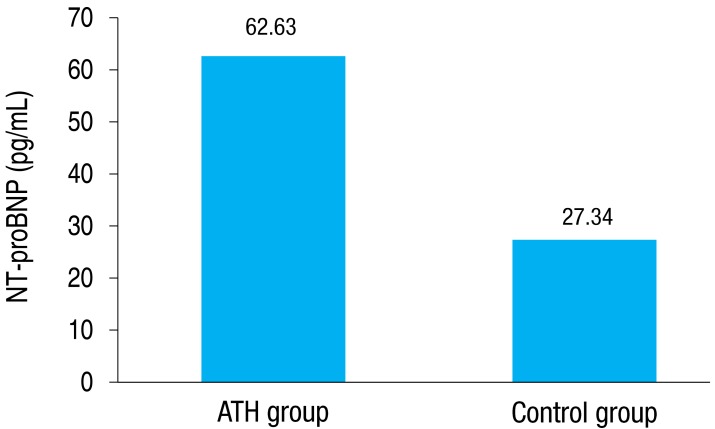
The snoring-tiredness during daytime-observed apnea-high blood pressure (STOP) questionnaire was completed by the patients' parents, and loud snoring was noted in the ATH group. The plasma NT-proBNP level was significantly higher in the ATH group than that in the controls (66.44┬▒37.63 pg/mL vs. 27.85┬▒8.89 pg/mL, P=0.001). The echocardiographic parameters were not significantly different between the groups.
Conclusion
We were unable to confirm the significance of echocardiographic evidence of RV dysfunction in the management of children with ATH. However, the plasma NT-proBNP level was significantly higher in the ATH group than that in the control, suggesting that chronic airway obstruction in children may carry a risk for cardiac dysfunction. Therefore, more patients should be examined using transthoracic echocardiography. In addition, pediatricians and otolaryngologists should consider cardiologic aspects during the management of children with severe ATH.
Adenotonsillar hypertrophy (ATH) is the most common cause of upper airway obstruction (UAO) in children. Moreover, it has been reported that chronic UAO can cause hypoxemia, hypercarbia-induced respiratory acidosis, and pulmonary vasoconstriction, which leads to cardiopulmonary complications such as right ventricle (RV) dysfunction, pulmonary hypertension and cor pulmonale1,2,3,4).
N-terminal of probrain natriuretic peptide (NT-proBNP) is a cardiac hormone secreted by the ventricular myocardium in response to ventricular volume expansion and pressure overload. It has been effectively used to diagnose RV dysfunction5,6,7).
To our knowledge, no definite guidelines are available to inform the decision of adenotonsillectomy in children with ATH. Therefore, we attempted to identify cardiologic and laboratory parameters that could be used for this purpose. In the present study, we evaluated cardiac performance in children with severe ATH on the basis of the plasma NT-proBNP level and conventional and Doppler echocardiography results.
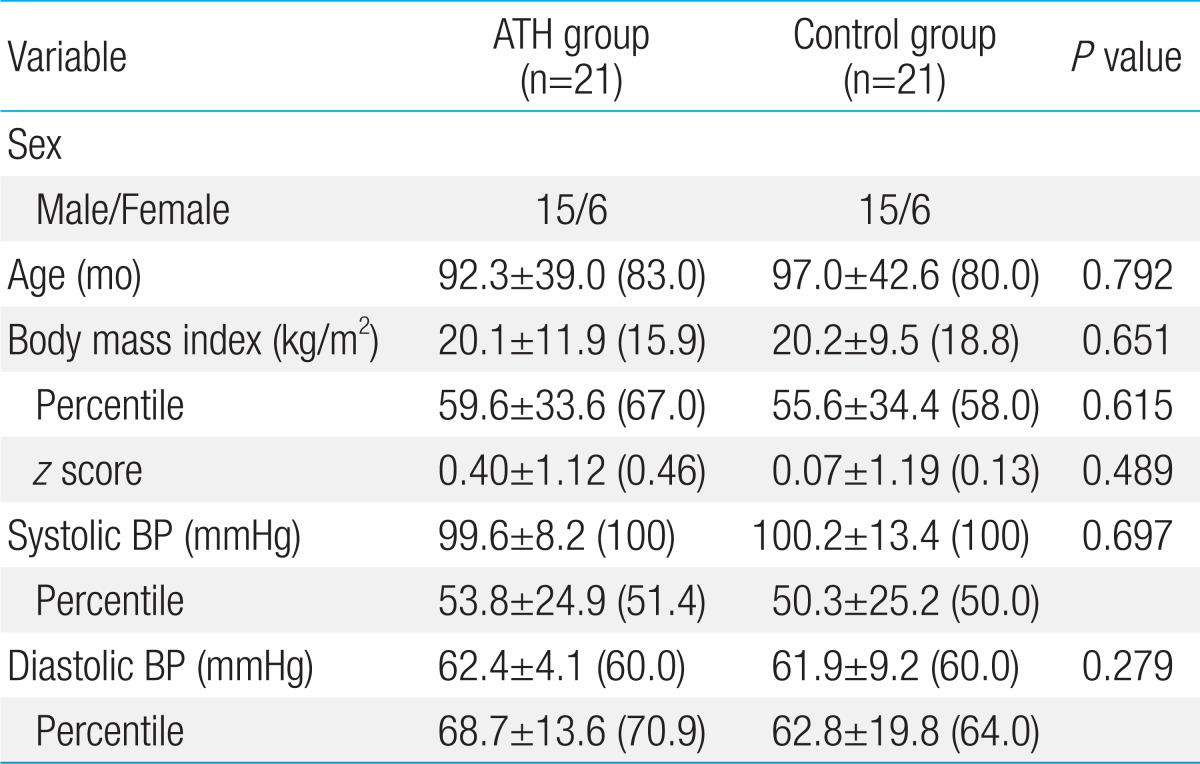
The present study was conducted at the Departments of Pediatric Cardiology and Otolaryngology, Konyang University Hospital, from June 2013 to October 2013. Twenty-one children (male/female, 15/6; mean age, 92.3┬▒39.0 months; age range, 4-15 years) with ATH and 21 healthy age- and gender-matched (male/female, 15/6; mean age, 97.0┬▒42.6 months; age range, 3-14 years) were included. Children in the control group were healthy and had no respiratory and cardiac symptoms. Otolaryngologists conducted flexible nasopharyngoscopy and lateral neck radiography for all patients. Hypertrophy of the adenoid and tonsils was graded according to the method of Cohen and Konak and on the Brodsky scale, respectively, as shown in Table 18,9). Patients with grade III or grade IV adenoid and tonsillar hypertrophy, defined as severe ATH, were enrolled in the study. Children with acute signs of infections, such as fever, leukocytosis, and increased C-reactive protein levels, which aggravate UAO were excluded.
The patients' parents filled out the snoring-tiredness during daytime-observed apnea-high blood pressure (STOP) questionnaire revised for children in the preoperative state. The STOP questionnaire is a self-reported, forced-choice (yes/no), paper-and-pencil scale. It includes the following four questions: S-"Do you snore loudly (louder than talking or loud enough to be heard through closed doors)?"; T-"Do you often feel tired, fatigued, or sleepy during the daytime?"; O-"Has anyone observed you stop breathing during your sleep?"; and P-"Do you have or are you being treated for high blood pressure?" If the answers are positive for two or more items, the questionnaire is scored as positive, indicating a high risk of obstructive sleep apnea (OSA), and these patients are considered to have severe ATH symptomatically10).
All children underwent transthoracic two-dimensional echocardiography conducted by a single pediatric cardiologist. The echocardiograms were obtained in the standard precordial positions. A Hewlett-Packard ultrasonic system with a pediatric probe (range, 3.5-5 MHz) was used for echocardiographic examination. The RV myocardial performance index (RVMPI) and tricuspid annular plane systolic excursion (TAPSE) were measured. RVMPI was calculated as described by Tei11) as the sum of the isovolumetric contraction and isovolumetric relaxation times divided by the ejection time. TAPSE was estimated using two-dimensional echo-guided M-mode recordings from the apical four-chamber view with the cursor placed on the free wall side of the tricuspid annulus12) and is presented as z score adjusted by age13). Random echocardiography recordings were analyzed to determine the intraobserver variability before the study. Intraobserver variabilities were <5% for all echocardiographic parameters.
Venous blood samples were drawn from all children and collected in nonheparinized tubes. Samples were centrifuged at 3,000 rpm for 10 minutes, and NT-proBNP was immediately assayed using Cobas test kits and the modular analytics E170 chemiluminescence immunoassay system.
Data were analyzed using the PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA). Continuous variables are presented as mean┬Ī┬Šstandard deviation (median). Moreover, the echocardiographic parameters and plasma NT-proBNP levels were categorized as either normal or abnormal, using the previously reported normal results as a reference14,15). The significance of the differences in the categorical variables between the groups was analyzed using chi-square analysis, whereas the significance of the differences in the continuous variables was evaluated using the Mann-Whitney U test. A P value<0.05 was considered significant.
The baseline characteristics of the ATH and control groups are shown in Table 2. The groups did not differ significantly in terms of age, sex, body mass index (BMI), and blood pressure (BP). The z score of BMI were slightly higher in the ATH group, but this difference was not statistically significant.
All ATH patients were reported to have a high risk of OSA, and the STOP questionnaire showed that all but one patient snored loudly and experienced sleep apnea.
The plasma NT-proBNP levels were significantly higher in the ATH group than the control group (66.44┬▒37.63 pg/mL [median, 62.63 pg/mL], range, 5.78-125.90 pg/mL vs. 27.85┬▒8.89 pg/mL [median, 27.34 pg/mL], range, 14.02-49.08 pg/mL; P=0.001) (Fig. 1).
The value of RVMPI was higher in the ATH group than the control group, but the difference was not statistically significant (P=0.186). Further, the values of the z score of TAPSE did not differ significantly between the groups (Table 3).
We determined the odds ratio (OR) for ATH by assessing abnormal results of echocardiographic and laboratory parameters representing RV function. ATH had no effect on the development of abnormal results of echocardiographic parameters such as the z score of TAPSE and RVMPI (OR, 0.56; 95% confidence interval [CI], 0.16-1.91 and OR, 8; 95% CI, 0.86-73.68, respectively). However, ATH patients were 22 times more likely to develop abnormal NT-proBNP levels than healthy controls were (OR, 22; 95% CI, 2.47-195.26; P=0.001) (Table 4).
The present study is a prospective patient-control study that aimed to evaluate cardiac dysfunction in children with chronic UAO on the basis of echocardiographic findings and the plasma level of NT-proBNP.
A previous study demonstrated that the size of both the adenoid and tonsil was significantly correlated with OSA and cardiopulmonary complications16,17). Therefore, we selected patients with grades III-IV of ATH confirmed using the method of Cohen and Konak and the Brodsky scale. Further, all ATH patients had severe symptoms associated with airway obstruction, as assessed using the STOP questionnaire.
It has been well known that sleep deprivation or poor quality sleep is associated with a higher rate of obesity18). Additionally, obesity has been shown to be an independent risk factor for UAO in children. However, it remains unclear whether the high body weight profile in the ATH group is the cause or result of chronic snoring19,20). Kocabas et al.21) documented that children with OSA have been higher in body weight profile than the controls. In other previous studies, there was not a statistically significant difference between the ATH group and control group in the BMI profiles7,22,23). The present study found similar BMI profiles in ATH patients as those reported previously, and the difference in the BMI profiles between the ATH group and control group in this study was also similar to those reported previously.
In this study, all subjects were evaluated using the echocardiographic parameters of RVMPI and TAPSE. Some previous studies have examined RV function in children with severe ATH by using these parameters. Duman et al.3) reported that the RVMPI, which reflects RV myocardial function, was significantly impaired in pediatric patients with advanced ATH. In our study, the RVMPI values in the ATH group were higher than those in the control group (0.39┬▒0.08 [median, 0.40] and 0.36┬▒0.10 [median, 0.33], respectively) but the difference was not statistically significance (P=0.186). Furthermore, the ATH patients were 8 times more likely to develop the abnormality of RVMPI values than the healthy controls, although there is not statistically significant (OR, 8; 95% CI, 0.86-73.68; P=0.093). Thus, further investigation with a large number of subjects is necessary to clarify the relationship between the echocardiographic parameters of RV dysfunction and ATH.
Significant ATH with respiratory symptoms such as snoring and sleep apnea is the main cause of upper mechanical airway obstruction in children. Chronic UAO is believed to lead to increased pulmonary artery pressure and pulmonary hypertension24,25). In addition to increased venous return and increased pulmonary resistance with ATH, the venous return to the right-side cardiac chambers is also increased, facilitated both by the decubitus horizontal position during sleep and by intrathoracic pressure which becomes more negative due to respiratory effort against the obstructive area. These kinds of changes can lead to an enlarged right atrium and ventricle and compromise ejection during systole26,27,28).
Previous studies have shown a relationship between ATH and pulmonary hypertension and RV dysfunction. In addition, many studies have indicated that the cardiopulmonary complications caused by ATH are improved following adenotonsillectomy29,30,31). Miman et al.32) documented full resolution of the symptoms of pulmonary hypertension after adenotonsillectomy in patients with pulmonary hypertension secondary to ATH. Furthermore, Duman et al.3) reported that the RVMPI was significantly impaired in pediatric patients with advanced ATH without evident cardiovascular disease, compared with age-matched control subjects, these cardiac changes were reversed following surgical intervention by adenotonsillectomy.
The usefulness of the plasma NT-proBNP level in assessment of RV function in children with congenital heart disease was reported by several studies33,34,35). In particular, plasma NT-proBNP levels can be used to detect RV systolic dysfunction in individuals with pulmonary hypertension. In the present study, we found that plasma NT-proBNP concentrations were significantly higher in the ATH group than the healthy controls (66.44┬▒37.63 pg/mL vs. 27.85┬▒8.89 pg/mL; P=0.001). Moreover, the plasma NT-proBNP levels are dependent on age. Therefore, we categorized the levels into two groups: within normal limits and above upper limits of normal compared with normal reference values based on age15). ATH patients were 22 times more likely to have abnormal NT-proBNP values than healthy controls (OR, 22; 95% CI, 2.47-195.26; P=0.001). This suggests that abnormal plasma NT-proBNP levels are an important indicator of RV dysfunction in the ATH group, and we speculated that this parameter may be helpful in evaluating disease severity and cardiac involvement in children with chronic UAO.
This study has certain limitations. First, the number of subjects included was relatively small. Second, we believe that the duration of snoring should also be considered as an important factor affecting the progression of cardiopulmonary compromise. However, although most parents of the ATH patients complained that their children had been snoring for a long time, we could not assess the exact duration of ATH. Third, this study could not evaluate adenoid and tonsillar grades in the control subjects, who might have mild to moderate ATH without significantly respiratory symptoms.
In conclusion, since no definite guidelines are available to inform the decision of adenotonsillectomy, we attempted to identify cardiologic and laboratory parameters that could be used for this purpose. Our results showed that the plasma level of NT-proBNP was significantly higher in the ATH group than the controls. However, we could not confirm echocardiographic evidence of RV dysfunction in children with chronic UAO. Thus, further investigations are required to determine the clinical importance of echocardiographic parameters in children with ATH. In addition, pediatricians and otolaryngologists should consider the cardiologic aspects during management of children with ATH.
Acknowledgments
This study was presented as a free paper in the Sixty-third Annual Fall Meeting of the Korean Pediatric Society, 2013 (Car-O-03).
References
1. Blum RH, McGowan FX Jr. Chronic upper airway obstruction and cardiac dysfunction: anatomy, pathophysiology and anesthetic implications. Paediatr Anaesth 2004;14:75ŌĆō83.


2. Brown OE, Manning SC, Ridenour B. Cor pulmonale secondary to tonsillar and adenoidal hypertrophy: management considerations. Int J Pediatr Otorhinolaryngol 1988;16:131ŌĆō139.


3. Duman D, Naiboglu B, Esen HS, Toros SZ, Demirtunc R. Impaired right ventricular function in adenotonsillar hypertrophy. Int J Cardiovasc Imaging 2008;24:261ŌĆō267.


4. Guilleminault C, Huang YS, Glamann C, Li K, Chan A. Adenotonsillectomy and obstructive sleep apnea in children: a prospective survey. Otolaryngol Head Neck Surg 2007;136:169ŌĆō175.


5. Krittayaphong R, Boonyasirinant T, Saiviroonporn P, Udompunturak S. NT-proBNP levels in the evaluation of right ventricular dysfunction in patients with coronary artery disease and abnormal left ventricular wall motion: a magnetic resonance imaging study. Coron Artery Dis 2008;19:481ŌĆō487.


6. Blyth KG, Groenning BA, Mark PB, Martin TN, Foster JE, Steedman T, et al. NT-proBNP can be used to detect right ventricular systolic dysfunction in pulmonary hypertension. Eur Respir J 2007;29:737ŌĆō744.


7. Oran B, Ozturk K, Cimen D, Vatansev H, Bulut S, Arslan D. Release of NT-pro brain natriuretic peptide in children before and after adenotonsillectomy. Int J Pediatr Otorhinolaryngol 2013;77:666ŌĆō669.


8. Cohen D, Konak S. The evaluation of radiographs of the nasopharynx. Clin Otolaryngol Allied Sci 1985;10:73ŌĆō78.


10. Chung F, Yegneswaran B, Liao P, Chung SA, Vairavanathan S, Islam S, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008;108:812ŌĆō821.


11. Tei C. New non-invasive index for combined systolic and diastolic ventricular function. J Cardiol 1995;26:135ŌĆō136.

12. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685ŌĆō713.


13. Koestenberger M, Ravekes W, Everett AD, Stueger HP, Heinzl B, Gamillscheg A, et al. Right ventricular function in infants, children and adolescents: reference values of the tricuspid annular plane systolic excursion (TAPSE) in 640 healthy patients and calculation of z score values. J Am Soc Echocardiogr 2009;22:715ŌĆō719.


14. Tei C, Dujardin KS, Hodge DO, Bailey KR, McGoon MD, Tajik AJ, et al. Doppler echocardiographic index for assessment of global right ventricular function. J Am Soc Echocardiogr 1996;9:838ŌĆō847.


15. Nir A, Lindinger A, Rauh M, Bar-Oz B, Laer S, Schwachtgen L, et al. NT-pro-B-type natriuretic peptide in infants and children: reference values based on combined data from four studies. Pediatr Cardiol 2009;30:3ŌĆō8.


16. Tatl─▒p─▒nar A, Biteker M, Meric K, Bayraktar GI, Tekkesin AI, Gokceer T. Adenotonsillar hypertrophy: correlation between obstruction types and cardiopulmonary complications. Laryngoscope 2012;122:676ŌĆō680.


17. Kang KT, Chou CH, Weng WC, Lee PL, Hsu WC. Associations between adenotonsillar hypertrophy, age, and obesity in children with obstructive sleep apnea. PLoS One 2013;8:e78666



18. Tasali E, Leproult R, Spiegel K. Reduced sleep duration or quality: relationships with insulin resistance and type 2 diabetes. Prog Cardiovasc Dis 2009;51:381ŌĆō391.


19. Kang KT, Lee PL, Weng WC, Hsu WC. Body weight status and obstructive sleep apnea in children. Int J Obes (Lond) 2012;36:920ŌĆō924.


20. Wing YK, Hui SH, Pak WM, Ho CK, Cheung A, Li AM, et al. A controlled study of sleep related disordered breathing in obese children. Arch Dis Child 2003;88:1043ŌĆō1047.



21. Kocabas A, Salman N, Ekici F, Cetin I, Akcan FA. Evaluation of cardiac functions and atrial electromechanical delay in children with adenotonsillar hypertrophy. Pediatr Cardiol 2014;35:785ŌĆō792.


22. Goldbart AD, Levitas A, Greenberg-Dotan S, Ben Shimol S, Broides A, Puterman M, et al. B-type natriuretic peptide and cardiovascular function in young children with obstructive sleep apnea. Chest 2010;138:528ŌĆō535.


23. Weber SA, Montovani JC, Matsubara B, Fioretto JR. Echocardiographic abnormalities in children with obstructive breathing disorders during sleep. J Pediatr (Rio J) 2007;83:518ŌĆō522.

24. Cayler GG, Johnson EE, Lewis BE, Kortzeborn JD, Jordan J, Fricker GA. Heart failure due to enlarged tonsils and adenoids. The cardiorespiratory syndrome of increased airway resistance. Am J Dis Child 1969;118:708ŌĆō717.


25. Sie KC, Perkins JA, Clarke WR. Acute right heart failure due to adenotonsillar hypertrophy. Int J Pediatr Otorhinolaryngol 1997;41:53ŌĆō58.


26. Phillips B. Sleep-disordered breathing and cardiovascular disease. Sleep Med Rev 2005;9:131ŌĆō140.


27. Steiner S, Strauer BE. Functional dynamics of the right ventricle and pulmonary circulation in obstructive sleep apnea Therapeutic consequences. Internist (Berl) 2004;45:1101ŌĆō1107.


28. Verrier RL, Harper RM, Hobson JA. Cardiovascular physiology: central and autonomic regulation. Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 3rd ed. Philadelphia: WB Saunders, 2000;:179ŌĆō191.
29. Yilmaz MD, Onrat E, Altuntas A, Kaya D, Kahveci OK, Ozel O, et al. The effects of tonsillectomy and adenoidectomy on pulmonary arterial pressure in children. Am J Otolaryngol 2005;26:18ŌĆō21.


30. Gerur K, Doven O, Unal M, Akkus N, Ozcan C. Preoperative and postoperative cardiac and clinical findings of patients with adenotonsillar hypertrophy. Int J Pediatr Otorhinolaryngol 2001;59:41ŌĆō46.


31. Jabbari Moghaddam Y, Bavil SG, Abavisani K. Do pre-adenotonsillectomy echocardiographic findings change postoperatively in children with severe adenotonsillar hypertrophy. J Saudi Heart Assoc 2011;23:31ŌĆō35.


32. Miman MC, Kirazli T, Ozyurek R. Doppler echocardiography in adenotonsillar hypertrophy. Int J Pediatr Otorhinolaryngol 2000;54:21ŌĆō26.


33. Sahin M, Portakal O, Karagoz T, Hascelik G, Ozkutlu S. Diagnostic performance of BNP and NT-ProBNP measurements in children with heart failure based on congenital heart defects and cardiomyopathies. Clin Biochem 2010;43:1278ŌĆō1281.


Fig.┬Ā1
Comparison of plasma NT-proBNP levels between the ATH and control groups. Values are presented as median. P=0.001. ATH, adenotonsillar hypertrophy; NT-proBNP, N-terminal of probrain natriuretic peptide.






 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


