|
Question: The immunogenicity and safety of GBP411 when administered to healthy infants are not understood.
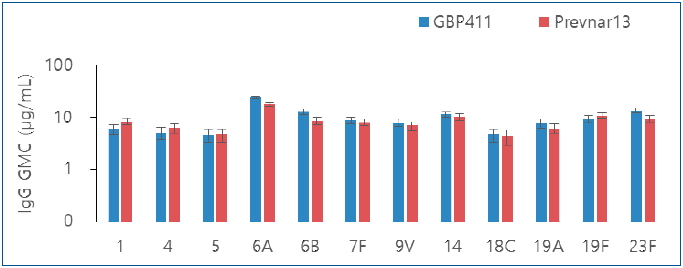
Finding: The intergroup differences were not significant for all 12 serotypes after the booster dose. The overall incidence of solicited local adverse events between the groups did not differ significantly.
Meaning: GBP411 with a 2p+1 dosing schedule induced a substantial immune response, and may be safe for administration to healthy infants. |


