< Previous Next >
Article Contents
| Clin Exp Pediatr > Volume 56(9); 2013 |
Abstract
A complex interplay between genetic and environmental factors partially contributes to the development of allergic diseases by affecting development during prenatal and early life. To explain the dramatic increase in the prevalence of allergic diseases, the hygiene hypothesis proposed that early exposure to infection prevented allergic diseases. The hygiene hypothesis has changed to the microbial hypothesis, in which exposure to microbes is closely linked to the development of the early immune system and allergic diseases. The intestinal flora may contribute to allergic disease through its substantial effect on mucosal immunity. Based on findings that exposure to microbial flora early in life can change the Th1/Th2 balance, thus favoring a Th1 cell response, probiotics may be beneficial in preventing allergic diseases. However, evidence from clinical and basic research to prove the efficacy of probiotics in preventing allergy is lacking. To date, studies have yielded inconsistent findings on the usefulness of probiotics in allergic diseases. It is difficult to demonstrate an exact effect of probiotics on asthma, allergic rhinitis, and food allergy because of study limitations, such as different first supplementation period, duration, different strains, short follow-up period, and host factors. However, many studies have demonstrated a significant clinical improvement in atopic dermatitis with the use of probiotics. An accurate understanding of the development of human immunity, intestinal barrier function, intestinal microbiota, and systemic immunity is required to comprehend the effects of probiotics on allergic diseases.
The Developmental Origins Hypothesis for Health and Disease proposes that all organ systems undergo developmental programming in utero that predetermines subsequent physiologic and metabolic adaptations during adult life1). Exposure to environmental factors during prenatal and early life can contribute to the development of allergic diseases, which affect human health later in life2). The rapid increase in the prevalence of allergic diseases and the high burden requires the development of new strategies for effective prevention, diagnosis, and treatment. In this regard, the hygiene and microbial hypothesis may be helpful. Because exposure to microbial flora early in life can change the balance of immune development, probiotics may be beneficial in preventing allergic diseases. In this article, we review the evidence supporting the hygiene hypothesis and the mechanisms of action of probiotics based on this hypothesis. We summarize recent clinical and basic research to elucidate the mechanisms of probiotics and their effects on allergic diseases.
The hygiene hypothesis was proposed more than 20 years ago by Strachan to explain the dramatic increase in the prevalence of allergic diseases over the past few decades3). The original hygiene hypothesis proposed that early exposure to infection through contact with older siblings prevented allergic diseases3,4). Later, the hygiene hypothesis was extended to explain the great increase in the prevalence of Th1 diseases, such as inflammatory bowel diseases and autoimmune diseases, that occurred over the same period5). However, the exact scientific underpinning for the hygiene hypothesis, for example, a specific infection that can prime the immune system to prevent disease, is an unsolved puzzle.
Several studies report a consistently low prevalence of allergies in farmers' children4); it remains unclear which specific factors are most important, but microbial exposure may play a role through the inhalation of endotoxin6). Endotoxin, a lipopolysaccharide present in the outer membrane of gram-negative bacteria, downregulates Th2 cytokine production7). However, in inner-city communities, domestic endotoxin exposure was positively associated with wheeze in children at 2 years of age8). One unanswered question is whether the greater levels of endotoxin associated with an inner-city lifestyle can explain the higher prevalence of asthma relative to that observed in rural areas.
Some studies have recently revisited the hygiene hypothesis and suggested that the decline in childhood infections is less important than how modern societal practices caused the disappearance of ancestral indigenous microbiota species that might confer benefits beyond our current understanding9). Initial studies of allergic diseases focused on gut microbiota. Allergic diseases among children are partially associated with differences in their intestinal microflora in countries with a low (Estonia) and high (Sweden) prevalence of allergies10). Evidences collected over the last few decades from human and mouse studies indicate that colonization of the gut early in life plays a substantial role in directing immune system development. Therefore, we need to understand the importance of gut colonization early in life and to determine whether altering microbial exposures during the perinatal period promotes lifelong dysregulated immune responses. In a mouse model, the intestinal bacterial flora was shown to play a crucial role in oral tolerance induction, probably by affecting the development of the immune system at the neonatal stage11).
After birth, the diversity of the gut microbiome increases with age12). A healthy human fetus is thought to develop within a bacteria-free environment. Upon birth, a neonate is exposed to a wide variety of microbes, many of which are encountered during and after passage through the birth canal. The latter comprises an ecosystem heavily colonized by a relatively limited set of bacterial taxa13). Vaginally delivered infants acquire bacterial communities resembling their mother's vaginal microbiota, which is dominated by Lactobacillus, Prevotella, or Sneathia spp., while infants delivered by caesarian section harbor bacterial communities similar to those found on the skin surface, which are dominated by Staphylococcus, Corynebacterium, and Propionibacterium species14).
Microbial exposure during the perinatal period is linked to the epigenetic regulation of genes involved in allergic inflammation, and it alters susceptibility to allergic diseases15). The emerging understanding of the importance of microbial contact during the fragile periods of fetal life, delivery, and infancy in healthy immune and metabolic programming, creates new opportunities to improve infant health and reduce the risk of disease in later life. According to the basis of the hygiene hypothesis, the microbial inhabitants of the human affect the early development of the immune system. This concept led us to investigate the probiotics-induced modulation of mechanisms underlying the development of allergic diseases.
Antibiotics enhance allergic airway responses in experimental animals by altering the intestinal microbiota, and probiotics modulate allergic responses in the lower respiratory tract16-20). In a double-blind, placebo-controlled study, 1,223 mothers with infants at high risk for allergy received a probiotic mixture (Bifidobacterium spp. and Propionibacteria spp.) or placebo during the last few months of pregnancy, and their infants received the same mixture from birth until 6 months of age. However, a preventive effect of probiotics on asthma was not observed up to 5 years of age20). In 2 other double-blind, placebo-controlled, randomized trials with infants at risk of allergy, probiotic supplementation failed to decrease the frequency of wheezing at 1 year and influence asthma prevalence rates at 2 years21,22). Supplementation with probiotics was associated with an increased rate of recurrent episodes of wheezy bronchitis23) but not with a reduced prevalence of inhalant allergen sensitization24). Probiotics prevented asthma-like symptoms in infants with atopic dermatitis25), whose pulmonary function and peak expiratory flow rates decreased significantly. In addition, the clinical symptom scores for asthma and allergic rhinitis decreased in the probiotic-treated patients26). Oral administration of probiotics attenuated the symptoms of allergic asthma in a mouse model, induced immune regulation by a CD4(+)CD25(+) Foxp3(+) regulatory T (Treg) cell-mediated mechanism16), and effectively suppressed airway hyperresponsiveness2).
Although the possibility of asthma prevention and treatment is indicated by research on animal models, no primary prevention study demonstrates an effect of probiotic supplementation in humans. Despite numerous studies, demonstration of an effect of probiotics has been impeded by limitations, such as different first supplementation periods, duration of supplementation, and short follow-up periods. To overcome these limitations, future studies should be conducted on larger numbers of subjects and for longer duration.
Immune responses in the gut may modulate immune responses in distant target organs, including the nose18,27). Probiotics alleviated nasal symptoms, prevented the pollen-induced infiltration of eosinophils into the nasal mucosa28), and modulated Th2-skewed immune responses in allergic rhinitis29).
Probiotics alleviated perennial and seasonal allergic rhinitis. In a study of preschoolers treated with probiotics or placebo for 12 months, there was a difference in the cumulative incidence of rhinitis episodes30). In adult patients with seasonal allergic rhinitis, probiotics modulated immune responses; they have the potential to alleviate the severity of symptoms31). However, other studies showed that probiotics provided few clinical benefits and did not alleviate the symptoms or reduce the use of medication32,33).
The heterogeneity of studies on the effects of probiotics in allergic rhinitis precludes meta-analysis. Unlike in other allergic diseases, the therapeutic effect of probiotics in allergic rhinitis has been primarily demonstrated, whereas their preventive effects have not been conclusively defined. Evidence indicates that allergic rhinitis may be subdivided into several phenotypes (perennial allergic rhinitis, seasonal allergic rhinitis, and Japanese cedar pollen-induced allergic rhinitis). Well-designed future studies that consider theses phenotypes of allergic rhinitis will help us to understand the effects of treatment on allergic rhinitis.
There are numerous studies on the efficacy of probiotics for the prevention and treatment of atopic dermatitis34,35) (Fig. 1). A Cochrane review showed that there was insufficient evidence to recommend the addition of probiotics to infant diets for the prevention of atopic dermatitis36). In contrast, there is convincing evidence that probiotics effectively prevent and treat adult and childhood atopic dermatitis37,38).
In most studies reporting beneficial effects, mothers received probiotics during pregnancy and their babies continued with the same product after birth. The preventive effects of probiotics on eczema were greater in infants with a family history of allergic diseases22,39,40).
A Korean population based study showed that prenatal and postnatal supplementation with a probiotic mixture effectively prevented the development of eczema in infants at high risk of allergy during the first year of life41). According to a review article, various meta-analyses and systematic reviews show that probiotics can prevent the development of atopic dermatitis, particularly in infants who were administered probiotics perinatally42). A study of probiotic supplementation in Korean adults with atopic dermatitis showed modified production of cytokines from the peripheral blood mononuclear cells of these patients35).
Oral administration of probiotics prevented the development of atopic dermatitis in a novel mouse model by suppressing the production of the inflammatory cytokines; interleukin (IL)-4 and thymic stromal lymphopoietin (TSLP) in the skin through a mechanism that may involve CD4(+)CD25(+)Foxp3(+) Treg cells43). Oral administration of Lactobacillus strains suppressed house-dust mite dermatitis in the NC/Nga mouse, a representative animal model of human atopic dermatitis44). A meta-analysis of the effect of probiotics on atopic dermatitis suggested that probiotics may be effective for preventing atopic dermatitis in high-risk infants. There is, however, insufficient evidence for giving probiotics to all pregnant women and their babies to prevent allergic diseases.
The authors of the Cochrane review advised caution in interpreting the results of their meta-analysis because of heterogeneity and high rates of follow-up loss (17-61%) in individual trials45). There is insufficient evidence that probiotics are clinically effective for treating atopic dermatitis; however, the suggestion that probiotics may be effective for preventing atopic dermatitis can be accepted under limited conditions. An accurate understanding of the development of human immunity, intestinal barrier function, intestinal microbiota, and systemic immunity is required to evaluate the effects of probiotics on allergic diseases such as atopic dermatitis. Further basic research into the roles and functions of probiotics will be required.
There are very few studies of probiotic treatment for food allergy, and no systematic review seems possible at present. Moreover, most of the clinical information regarding probiotics and food allergy is derived from studies of patients with atopic dermatitis. The therapeutic oral administration of probiotics suppressed the skewed Th2 response in allergic mice and protected against anaphylactic reactions in recent murine models of food allergy46,47).
In several clinical trials, however, oral supplementation with probiotics did not significantly affect allergic inflammatory markers (total and specific immunoglobulin E) or cytokines; there were no positive therapeutic outcomes (improvement of symptoms and acquisition of tolerance)48,49).
In studies reporting beneficial therapeutic effects, most infants were exclusively breast-fed or provided an extensively hydrolyzed formula, and the effects reported were related to atopic dermatitis rather than food allergy50,51). The most significant improvements were observed when probiotics were used in conjunction with breastfeeding or a hypoallergenic formula.
To date, studies have yielded inconsistent findings on the efficacy of probiotics for preventing and treating food allergy. These discrepancies may be attributed to variations in multiple factors related to the probiotics (type, dose, mixtures, and duration) or to the recipients (birth method, diet, and age). Therefore, further studies are required to overcome these limitations.
Accumulating data suggest that certain bacterial strains provide protective signals, while others strains may stimulate aggressive and damaging immune responses. The effects on an individual's microbiota of the continuous administration of probiotics from an early age, which stimulates the immune system, has been considered beneficial by many clinicians and researchers (Fig. 2). However, the results of many studies of allergic diseases are not consistent. In particular, studies of allergic diseases in humans have not established any definite beneficial effects of probiotics. In this section, we discuss the mechanism of action of probiotics and summarize recent clinical data and animal studies on the effects of probiotics on allergic diseases (Table 1).
The pathogenesis of allergic diseases was first described as an imbalance of Th1/Th2 cells. Probiotics inhibit allergic diseases by suppressing the Th2 response. Interleukin (IL)-4, IL-5, and IL-13 are representative cytokines released by Th2 cells that are suppressed by probiotics16,52) (Fig. 3). Further, probiotics increase IL-10 and tumor growth factor (TGF)-β levels by inducing Tregs cells in allergic diseases52). However, the results of many studies differ, and they cannot be fully explained by an imbalance of Th1/Th2 responses in the development of allergic diseases. Consequently, the mechanism of probiotics is open to dispute.
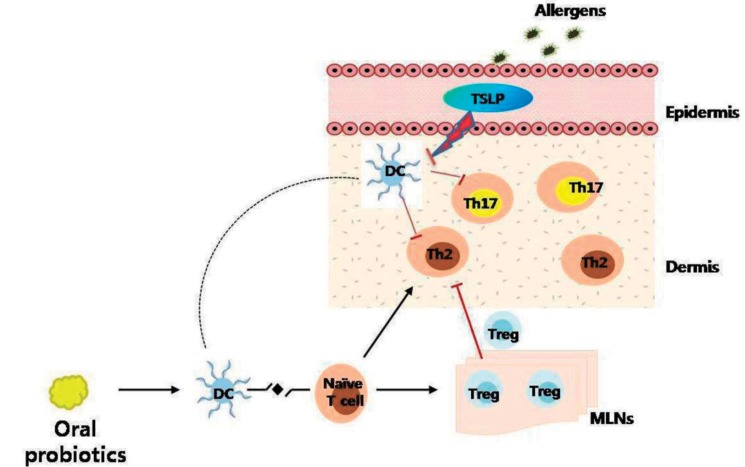
The previously discussed mechanisms of probiotics were superseded following the discovery of a novel paradigm in the development of allergic diseases. Th17 cells induce allergic inflammation in a mouse model of asthma53); probiotics suppressed this inflammation52). Probiotics suppressed interferon-γ (Th1-related cytokines), IL-4 (Th2-related cytokines), and Th17 cells in splenic CD4 T cells and increased the expression of IL-10 and TGF-β (Treg-related cytokines) in mesenteric lymph nodes54). Our own studies using a mouse model of atopic dermatitis demonstrated the suppression of the Th17 response in the skin following the administration of probiotics (Fig. 4).
The influence of probiotics on T cell differentiation suggests that dendritic cells (DCs) play an important role in the mechanism of action of probiotics in allergic diseases54,55). Thus, it is reasonable to conclude that probiotics inhibit naive T cell differentiation to Th2 cells. Moreover, probiotics inhibit the differentiation of mature DCs in vitro54,55). However, very few in vivo studies of allergic diseases have demonstrated a relationship between mature DCs and probiotics. In addition, some studies have shown this effect only in tumors19). Our research confirms that DCs are involved in the suppression of asthma by probiotics following the transfer of mature DCs in mice56).
TSLP plays an important role in the DC-mediated differentiation of T cells55). We are focusing on how Th2, Th17, and Treg cells along with TSLP influence the effects of probiotics on allergic diseases. However, further studies are needed to identify the additional mechanisms of probiotics on allergic diseases in humans and animal models.
Examining interactions between microorganisms in the environment, microorganisms in the mucosa, and humans and assessing how microorganisms modulate immune responses may be an interesting new approach in studying human diseases caused by chronic inflammation. The interactions between probiotics and the immune system might be beneficial for preventing allergic disease; however, evidence is lacking to support this conclusion. Moreover, the advent of the microbial hypothesis has raised new concerns about the association between immune responses and human gut microbiota and the impact of this association on the development of allergic diseases. Future research based on extending this concept may help to improve the efficacy of probiotics for preventing and treating allergic diseases, particularly atopic dermatitis. Confirmation of the association between the gut microbiota and the development of allergic diseases in humans awaits future research.
Acknowledgments
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (2012-0007128).
References
1. Waterland RA, Michels KB. Epigenetic epidemiology of the developmental origins hypothesis. Annu Rev Nutr 2007;27:363–388.


2. Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med 2009;27:358–368.



3. Strachan DP. Family size, infection and atopy: the first decade of the "hygiene hypothesis". Thorax 2000;55(Suppl 1): S2–S10.



4. Lee SY, Kwon JW, Seo JH, Song YH, Kim BJ, Yu J, et al. Prevalence of atopy and allergic diseases in Korean children: associations with a farming environment and rural lifestyle. Int Arch Allergy Immunol 2012;158:168–174.


6. Lee E, Lee SY, Ahn KM, Kim KW, Shin YH, Hong SJ. Interactions between CD14/IL-13 genes and caesarean section delivery may affect the development of atopic dermatitis in a COCOA study In: Poster presented at: 2013 Annual Meeting of AAAAI. 2013 Feb 22-26; San Diego, USA. Milwaukee, American Academy of Allergy Asthma & Immunology. 2013.
7. Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med 2002;196:1645–1651.



8. Perzanowski MS, Miller RL, Thorne PS, Barr RG, Divjan A, Sheares BJ, et al. Endotoxin in inner-city homes: associations with wheeze and eczema in early childhood. J Allergy Clin Immunol 2006;117:1082–1089.



9. Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nat Rev Microbiol 2009;7:887–894.



10. Iacono A, Raso GM, Canani RB, Calignano A, Meli R. Probiotics as an emerging therapeutic strategy to treat NAFLD: focus on molecular and biochemical mechanisms. J Nutr Biochem 2011;22:699–711.


11. Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 2002;347:911–920.


12. Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature 2012;486:222–227.



13. Hyman RW, Fukushima M, Diamond L, Kumm J, Giudice LC, Davis RW. Microbes on the human vaginal epithelium. Proc Natl Acad Sci U S A 2005;102:7952–7957.



14. Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A 2010;107:11971–11975.



15. Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science 2012;336:489–493.



16. Jang SO, Kim HJ, Kim YJ, Kang MJ, Kwon JW, Seo JH, et al. Asthma prevention by Lactobacillus rhamnosus in a mouse model is associated with CD4(+)CD25(+)Foxp3(+) T cells. Allergy Asthma Immunol Res 2012;4:150–156.



17. Pelucchi C, Chatenoud L, Turati F, Galeone C, Moja L, Bach JF, et al. Probiotics supplementation during pregnancy or infancy for the prevention of atopic dermatitis: a meta-analysis. Epidemiology 2012;23:402–414.


18. Wold AE. The hygiene hypothesis revised: is the rising frequency of allergy due to changes in the intestinal flora? Allergy 1998;53(46 Suppl): 20–25.

19. Chen CC, Chiu CH, Lin TY, Shi HN, Walker WA. Effect of probiotics Lactobacillus acidophilus on Citrobacter rodentium colitis: the role of dendritic cells. Pediatr Res 2009;65:169–175.



20. Kuitunen M, Kukkonen K, Juntunen-Backman K, Korpela R, Poussa T, Tuure T, et al. Probiotics prevent IgE-associated allergy until age 5 years in cesarean-delivered children but not in the total cohort. J Allergy Clin Immunol 2009;123:335–341.


21. Taylor AL, Dunstan JA, Prescott SL. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: a randomized controlled trial. J Allergy Clin Immunol 2007;119:184–191.


22. Abrahamsson TR, Jakobsson T, Bottcher MF, Fredrikson M, Jenmalm MC, Bjorksten B, et al. Probiotics in prevention of IgE-associated eczema: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol 2007;119:1174–1180.


23. Kopp MV, Hennemuth I, Heinzmann A, Urbanek R. Randomized, double-blind, placebo-controlled trial of probiotics for primary prevention: no clinical effects of Lactobacillus GG supplementation. Pediatrics 2008;121:e850–e856.


24. Kalliomäki M, Salminen S, Poussa T, Arvilommi H, Isolauri E. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet 2003;361:1869–1871.


25. van der Aa LB, van Aalderen WM, Heymans HS, Henk Sillevis Smitt J, Nauta AJ, Knippels LM, et al. Synbiotics prevent asthma-like symptoms in infants with atopic dermatitis. Allergy 2011;66:170–177.


26. Chen YS, Jan RL, Lin YL, Chen HH, Wang JY. Randomized placebo-controlled trial of lactobacillus on asthmatic children with allergic rhinitis. Pediatr Pulmonol 2010;45:1111–1120.


27. Lenoir-Wijnkoop I, Sanders ME, Cabana MD, Caglar E, Corthier G, Rayes N, et al. Probiotic and prebiotic influence beyond the intestinal tract. Nutr Rev 2007;65:469–489.


28. Ouwehand AC, Nermes M, Collado MC, Rautonen N, Salminen S, Isolauri E. Specific probiotics alleviate allergic rhinitis during the birch pollen season. World J Gastroenterol 2009;15:3261–3268.



29. Wassenberg J, Nutten S, Audran R, Barbier N, Aubert V, Moulin J, et al. Effect of Lactobacillus paracasei ST11 on a nasal provocation test with grass pollen in allergic rhinitis. Clin Exp Allergy 2011;41:565–573.


30. Rose MA, Stieglitz F, Koksal A, Schubert R, Schulze J, Zielen S. Efficacy of probiotic Lactobacillus GG on allergic sensitization and asthma in infants at risk. Clin Exp Allergy 2010;40:1398–1405.


31. Ivory K, Chambers SJ, Pin C, Prieto E, Arques JL, Nicoletti C. Oral delivery of Lactobacillus casei Shirota modifies allergen-induced immune responses in allergic rhinitis. Clin Exp Allergy 2008;38:1282–1289.


32. Helin T, Haahtela S, Haahtela T. No effect of oral treatment with an intestinal bacterial strain, Lactobacillus rhamnosus (ATCC 53103), on birch-pollen allergy: a placebo-controlled double-blind study. Allergy 2002;57:243–246.


33. Koyama T, Kirjavainen PV, Fisher C, Anukam K, Summers K, Hekmat S, et al. Development and pilot evaluation of a novel probiotic mixture for the management of seasonal allergic rhinitis. Can J Microbiol 2010;56:730–738.


34. Fiocchi A, Burks W, Bahna SL, Bielory L, Boyle RJ, Cocco R, et al. Clinical use of probiotics in pediatric allergy (CUPPA): a world allergy organization position paper. World Allergy Organ J 2012;5:148–167.



35. Kim H, Kwack K, Kim DY, Ji GE. Oral probiotic bacterial administration suppressed allergic responses in an ovalbumin-induced allergy mouse model. FEMS Immunol Med Microbiol 2005;45:259–267.


36. Osborn DA, Sinn JK. Probiotics in infants for prevention of allergic disease and food hypersensitivity. Cochrane Database Syst Rev 2007;(4): CD006475

37. Drago L, Iemoli E, Rodighiero V, Nicola L, De Vecchi E, Piconi S. Effects of Lactobacillus salivarius LS01 (DSM 22775) treatment on adult atopic dermatitis: a randomized placebo-controlled study. Int J Immunopathol Pharmacol 2011;24:1037–1048.


38. Lee J, Seto D, Bielory L. Meta-analysis of clinical trials of probiotics for prevention and treatment of pediatric atopic dermatitis. J Allergy Clin Immunol 2008;121:116–121.e11.


39. Kukkonen K, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, Poussa T, et al. Probiotics and prebiotic galacto-oligosaccharides in the prevention of allergic diseases: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol 2007;119:192–198.


40. Jenmalm MC, Duchen K. Timing of allergy-preventive and immunomodulatory dietary interventions - are prenatal, perinatal or postnatal strategies optimal? Clin Exp Allergy 2013;43:273–278.


41. Kim JY, Kwon JH, Ahn SH, Lee SI, Han YS, Choi YO, et al. Effect of probiotic mix (Bifidobacterium bifidum, Bifidobacterium lactis, Lactobacillus acidophilus) in the primary prevention of eczema: a double-blind, randomized, placebo-controlled trial. Pediatr Allergy Immunol 2010;21(2 Pt 2): e386–e393.


42. Kim NY, Ji GE. Effects of probiotics on the prevention of atopic dermatitis. Korean J Pediatr 2012;55:193–201.



43. Kim HJ, Kim YJ, Kang MJ, Seo JH, Kim HY, Jeong SK, et al. A novel mouse model of atopic dermatitis with epicutaneous allergen sensitization and the effect of Lactobacillus rhamnosus. Exp Dermatol 2012;21:672–675.


44. Won TJ, Kim B, Lim YT, Song DS, Park SY, Park ES, et al. Oral administration of Lactobacillus strains from Kimchi inhibits atopic dermatitis in NC/Nga mice. J Appl Microbiol 2011;110:1195–1202.


45. Nowak-Wegrzyn A, Muraro A. Food allergy therapy: is a cure within reach? Pediatr Clin North Am 2011;58:511–530. xii


46. Zhang LL, Chen X, Zheng PY, Luo Y, Lu GF, Liu ZQ, et al. Oral Bifidobacterium modulates intestinal immune inflammation in mice with food allergy. J Gastroenterol Hepatol 2010;25:928–934.


47. Schiavi E, Barletta B, Butteroni C, Corinti S, Boirivant M, Di Felice G. Oral therapeutic administration of a probiotic mixture suppresses established Th2 responses and systemic anaphylaxis in a murine model of food allergy. Allergy 2011;66:499–508.


48. Brouwer ML, Wolt-Plompen SA, Dubois AE, van der Heide S, Jansen DF, Hoijer MA, et al. No effects of probiotics on atopic dermatitis in infancy: a randomized placebo-controlled trial. Clin Exp Allergy 2006;36:899–906.


49. Hol J, van Leer EH, Elink Schuurman BE, de Ruiter LF, Samsom JN, Hop W, et al. The acquisition of tolerance toward cow's milk through probiotic supplementation: a randomized, controlled trial. J Allergy Clin Immunol 2008;121:1448–1454.


50. Majamaa H, Isolauri E. Probiotics: a novel approach in the management of food allergy. J Allergy Clin Immunol 1997;99:179–185.


51. Isolauri E, Arvola T, Sutas Y, Moilanen E, Salminen S. Probiotics in the management of atopic eczema. Clin Exp Allergy 2000;30:1604–1610.

52. Feleszko W, Jaworska J, Rha RD, Steinhausen S, Avagyan A, Jaudszus A, et al. Probiotic-induced suppression of allergic sensitization and airway inflammation is associated with an increase of T regulatory-dependent mechanisms in a murine model of asthma. Clin Exp Allergy 2007;37:498–505.


53. Jan RL, Yeh KC, Hsieh MH, Lin YL, Kao HF, Li PH, et al. Lactobacillus gasseri suppresses Th17 pro-inflammatory response and attenuates allergen-induced airway inflammation in a mouse model of allergic asthma. Br J Nutr 2012;108:130–139.


54. Kwon HK, Lee CG, So JS, Chae CS, Hwang JS, Sahoo A, et al. Generation of regulatory dendritic cells and CD4+Foxp3+ T cells by probiotics administration suppresses immune disorders. Proc Natl Acad Sci U S A 2010;107:2159–2164.



55. Weiss G, Christensen HR, Zeuthen LH, Vogensen FK, Jakobsen M, Frokiær H. Lactobacilli and bifidobacteria induce differential interferon-β profiles in dendritic cells. Cytokine 2011;56:520–530.


56. Kim HJ, Kim YJ, Lee SH, Kang MJ, Yu HS, Jung YH, et al. Effects of Lactobacillus rhamnosus on asthma with an adoptive transfer of dendritic cells in mice. J Appl Microbiol 2013;6 03 [Epub]. http://dx.doi.org/10.1111/jam.12268.
57. Lee SY, Lee JY, Jang SO, Kang MJ, Song YH, Kim BJ, et al. The immunologic effects of Lactobacillus rhamnosus (Lcr35) supplements in adult patients with atopic dermatitis. Korean J Asthma Allergy Clin Immunol 2008;28:128–133.
58. Kong DY, Yang HJ, Pyun BY. The effects on treatment of atopic dermatitis with oral Lactobacillus casei supplements in Korean children. Pediatr Allergy Respir Dis 2007;17:27–37.
59. Yu J, Jang SO, Kim BJ, Song YH, Kwon JW, Kang MJ, et al. The effects of Lactobacillus rhamnosus on the prevention of asthma in a murine model. Allergy Asthma Immunol Res 2010;2:199–205.



60. Hong HJ, Kim E, Cho D, Kim TS. Differential suppression of heat-killed lactobacilli isolated from kimchi, a Korean traditional food, on airway hyper-responsiveness in mice. J Clin Immunol 2010;30:449–458.


61. Kim JY, Choi YO, Ji GE. Effect of oral probiotics (Bifidobacterium lactis AD011 and Lactobacillus acidophilus AD031) administration on ovalbumin-induced food allergy mouse model. J Microbiol Biotechnol 2008;18:1393–1400.

62. Choi IS, Lim YJ, Na HS, Lee HC, Choung JT, Kook H. The effect of Lactobacillus acidophilus on the Primary prevention of asthma in a murine asthmatic model. Pediatr Allergy Respir Dis 2008;18:208–218.
Fig. 1
Usefulness of probiotics in the prevention of atopic dermatitis. The preventive effect of probiotics on atopic dermatitis (A) and immunoglobulin E-associated atopic dermatitis (B). RR, relative risk; CI, confidence interval. Reproduced from Pelucchi C, et al. Epidemiology 2012;23:402-14, with permission of Lippincott Williams & Wilkins, Inc.17).
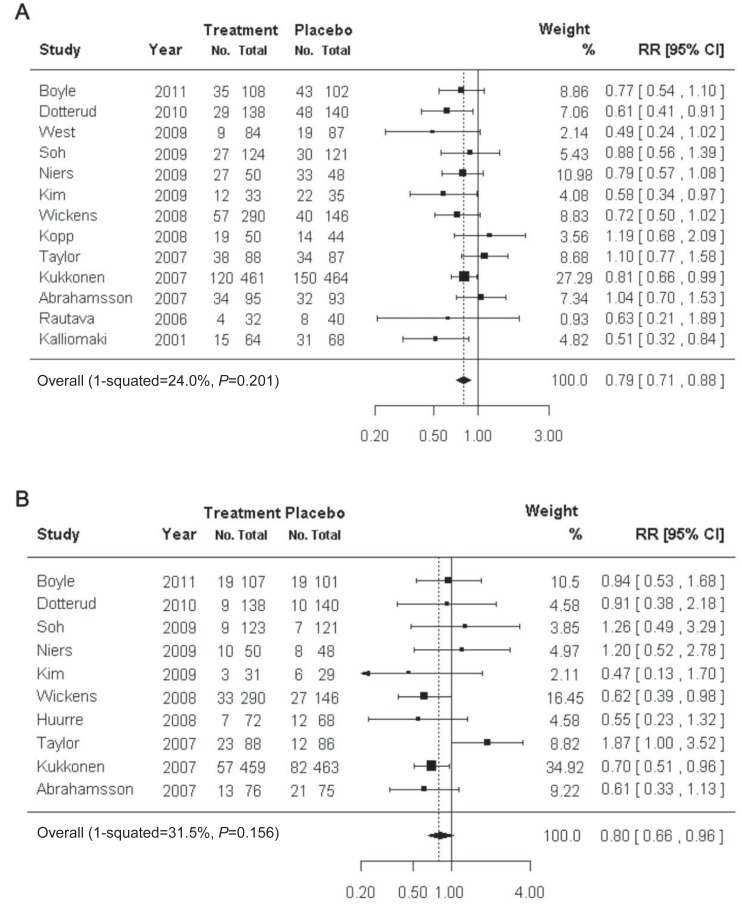
Fig. 3
Immune mechanisms of probiotics. DC, dendritic cells; TJ, tight junction; SCFA, short chain fatty acid; IgA, immunoglobulin A; IgE, immunoglobulin E; IL, interleukin; TNF, tumor necrosis factor; IFN, interferon; NF-κB, nuclear factor-κB; ROS, reactive oxygen species. Reproduced from Iacono A, et al. J Nutr Biochem 2011;22:699-711, with permission of Elsevier Inc.10).
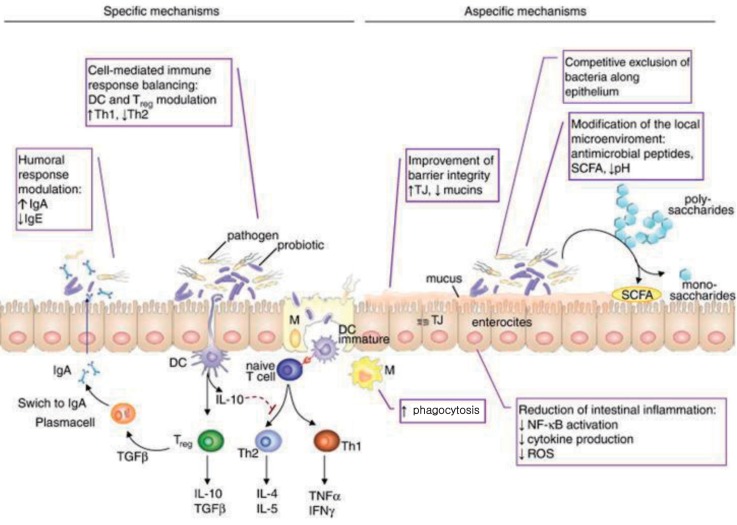
Fig. 4
Proposed mechanism of probiotics in an animal model of atopic dermatitis. Exposure to allergens on atopic skin increases the expression of thymic stromal lymphopoietin (TSLP) in the skin. TSLP-stimulated dendritic cells (DCs) induce naive T cells to differentiate into Th2 cells and Th17 cells, which induce allergic inflammation in the skin. Oral probiotics might inhibit these processes by increasing the number of regulatory T (Treg) cells in mesenteric lymph nodes. Tregs cells then migrate to the skin from the lymph nodes through lymphatic drainage, and suppress Th2 and Th17 responses (┴) in the skin. Tregs cells also suppress the expression of TSLP in the skin (┴). MLNs, mesenteric lymph nodes.




 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


