Introduction
Listeria monocytogenes is a gram-positive rod that is often found in the natural environment including the soil and rivers, wild animals, and livestock, and usually causes human infection through contaminated food. The human listeria infections transmitted by food are different from the enteritis caused by other bacteria. The infections are usually found in immunocompromised patients, pregnant women, infants and babies; listeria is very rare in healthy children1-3). Listeria causes central nervous system infections, endocarditis, and sepsis in patients with organ transplants, patients receiving immunosuppressive therapy, and patients with malignancies. An infection during pregnancy can cause miscarriage, sepsis, and premature birth, as well as amnionitis, disseminated abscess accompanied by sepsis, granuloma, and late-onset meningitis, conjunctivitis, and pneumonia4). Listeria infection in infants usually causes meningoencephalitis, conjunctivitis, and pneumonia; it primarily invades the brain stem rather than supratentorial areas, and is often accompanied by complications such as acute hydrocephalus. The clinical symptoms are similar to other cases of meningoencephalitis caused by bacteria including: fever, headache, vomiting, and altered mental status. Other forms of central nervous system infection include: encephalitis, brain abscess, and spine abscess. In addition, listeria can cause a suppurative arthritis, osteomyelitis, and liver abscess3).
According to prior reports up to the present, infections by listeria are rare in Korea5); however, infections in healthy children without immune system abnormalities are very rare6). Here we report a rare case of meningoencephalitis caused by L. monocytogenes in a previously healthy 7-year-old girl.
Case report
A previously healthy 7-year-old girl presented with periodic fever of over 38Ōäā, headache, abdominal pain, and watery diarrhea for six days; she had projectile vomiting and a severe headache accompanied by lethargy, and was admitted to the hospital. She had taken the conjugated pneumococcal vaccines and Haemophilus influenzae type b vaccines. She had never been contacted with contaminated foods and domestic animals. Although the mental status was intact at the time of presentation, neck stiffness, the Bruzinski sign and Kernig sign were all positive; there was no abnormality in motor function and muscle strength, and sensation was intact. The deep tendon reflexes of the lower limbs were hyper-reactive. The laboratory tests included: white blood cell counts 21,150/┬ĄL (segmented neutrophils 89%, lymphocytes 6.3%), ESR 57 mm/hr, CRP 9.52 mg/dL, and the fasting blood sugar was 169 mg/dL. The cerebrospinal fluid (CSF) examination showed 1,500/┬ĄL white blood cells (segmented neutrophils 30%), 250/┬ĄL red blood cells, a sugar of 25 mg/dL, and protein of 117 mg/dL; there were no bacteria noted on gram staining. PCR for tuberculosis, the herpes simplex virus antibody test, the co-agglutination test, and cryptococcus antigen test were all negative. We started antibiotic treatment with ceftriaxone and amikacin along with steroid injections before receiving the results of the cultures.
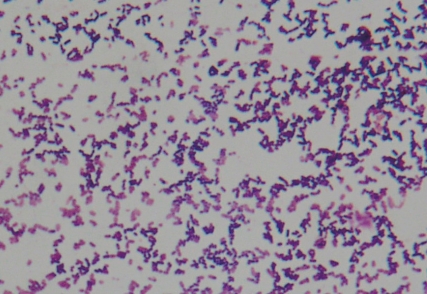
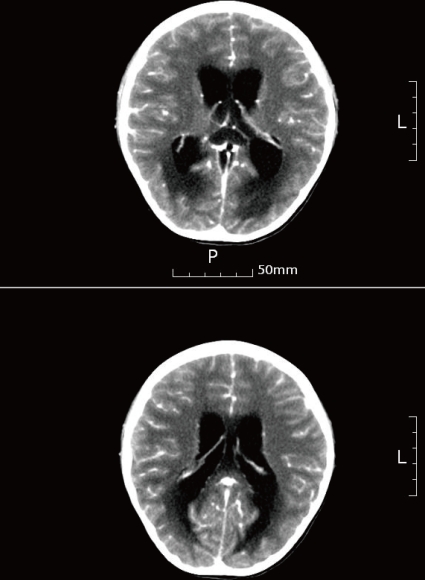
On the second hospital day, we added vancomycin to the treatment because of continued fever of over 39Ōäā, severe headache, vomiting, and altered mental status; the patient appeared stuporous and delirious. A brain MRI was performed because of a suspected increase in the intracranial pressure and the development of diplopia; there were no clinical findings suggestive of brain edema, such as an increase in the size of the ventricles. On the third hospital day, the fever and headache continued, and the patient complained of pain in both legs. Due to the worsening of the mental status, we repeated the CSF examination and blood tests. The results of the blood test showed 37,860/┬ĄL white blood cells (segmented neutrophils 88.2%, lymphocytes 5.3%), an ESR of 50 mm/hr, CRP 17.19 mg/dL, and fasting blood sugar of 142 mg/dL. The CSF had a white blood cell count of 2,000/┬ĄL (segmented neutrophils 35%), red blood cell count of 250/┬ĄL, a sugar of 73 mg/dL, protein 319 mg/dL, and gram-positive rods were confirmed on gram staining. L. monocytogenes was identified in both the blood and CSF, and ampicillin was added to the treatment (Fig. 1). The antimicrobial susceptibility test showed that the organisms were sensitive to vancomycin, ampicillin, and aminoglycosides. Testing of immunity included: an IgG of 872 mg/dL, IgA 208 mg/dL, IgM 257 mg/dL, and IgE 960 IU/mL; except for the increase in IgE, the C3 113 mg/dL, C4 28.2 mg/dL, and CH50 51.1 U/mL were all within normal range. The CD3 was 61.3% (1798/mm3), CD4 32.7% (959/mm3), CD8 35.2% (1033/mm3), and CD19 17% (499/mm3) all within normal limits. In addition, an ultrasound of the thymus was within normal limits. On the fourth hospital day, although the patient responded to pain stimulation, her conversation was confused and meaningless. In addition, there was no voluntary movement. The pupillary reflexes were slow, and nystagmus was noted; in addition, the muscle strength decreased to grade 2. The patient developed respiratory difficulty and her state of alertness was at the semicoma level. The patient was transferred to the intensive care unit where ventilator support was initiated. CT of the brain, showed hydrocephalus, parenchymal edema, and brain stem extrusion along with an increase in the third and fourth cerebral and lateral ventricles; extraventricular drainage was performed (Fig. 2). Additional extraventricular drainage, two consecutive times, was performed on the sixth and fourteenth day. On the 17th hospital day, the CSF examination showed 32/┬ĄL white blood cells, 3,100/┬ĄL red blood cells, a sugar of 99 mg/dL, and protein of 169 mg/dL; there were no bacteria detected on gram staining. No bacteria were found on the blood and CSF cultures. Due to the worsening hydrocephalus noted on the brain CT and because the level of consciousness was not improving a ventriculoperitoneal shunt procedure was performed on the 22nd hospital day. On the 25th hospital day, the patient could be taken off of ventilator support. She responded appropriately and her language was no longer confused. On the 31st hospital day, the vital signs were stable, and she was transferred from the intensive care unit to the general ward. The brain CT, on the 38th hospital day, showed a communicating hydrocephalus with ventriculostomy and parenchymal edema.
The child recovered consciousness and motor functions and was discharged after 61 days in the hospital.
Discussion
L. monocytogenes is an anaerobic gram-positive bacillus that is usually found in the soil, dust, fertilizer, sewage, river water, plants, and food, as well as animal intestines. Meningoencephalitis caused by L. monocytogenes usually occurs in patients with neoplastic disease, those taking high doses of steroids, the elderly with weak immunity, pregnant women, and infants1-3). Listeria infections are frequently transmitted through food. Listeria infections are associated with sepsis, meningoencephalitis, and granulomas7, 8). When infection due to listeria occurs, it can be fatal; the mortality rate is up to 30%.
L. monocytogenes enters a variety of host cells such as macrophages, epithelial cells, and parenchymal cells, by phagocytosis. Listeria invades the phagocytic vacuole, and multiplies inside of the cell. It forms a structure that is wrapped around by actin filament from the host cell in the protoplasm. This results in a protrusion of the membrane of the host cell, and adhesion to nearby cells, facilitating bacterial transmission among cells. As a result, the listeria can hide inside of the host cell and be protected from the immune reaction of host cells, enabling the spread of infection9). L. monocytogenes invasion has a predilection for endothelial cells in brain microvessels, and can cause severe sequelae by affecting white blood cells and stimulating an inflammatory reaction within the central nervous system10).
Meningoencephalitis caused by listeria in healthy children is very rare1). A temporary change in the T-lymphocyte population during the latent period of bacterial infection, has been reported in cases where meningoencephalitis caused by listeria occurred in healthy children1, 2). Cases with meningitis due to virus, bacteria, parasites, or mold that causes acute hydrocephalus as a secondary complication are uncommon1, 11). However, according to previously reported cases, where meningoencephalitis caused by listeria occurred in healthy children, the disease progressed rapidly and was associated with acute hydrocephalus; in these cases there was a high mortality rate1). Therefore, rapid diagnosis and treatment are important for the prevention of serious complications. There have been many cases where listeria infections were mistaken for streptococcus pneumonia or diphtheria, which also has a low positive rate of gram staining. A listeria infection must be included in the differential diagnosis of meningoencephalitis, even though such an infection is rare in healthy children without immune function disorders1, 3, 4, 12). In this case, although we were unable to identify listeria strains from the initial gram stain of the CSF, we identified listeria, as a gram-positive bacillus, on the second CSF gram stain, and have identified L. monocytogenes from the blood and CSF cultures. Therefore, repeat CSF studies should be performed in cases with meningoencephalitis and no specific organism identified on initial evaluations1, 3, 9).
The antibiotics used to treat meningoencephalitis caused by listeria include ampicillin and penicillin; use combined with aminoglycoisides such as gentamicin and amikacin have been reported to be effective5, 10, 13, 14). Penicillin resistant strains can be treated using trimethoprim-sulfamethoxazole13). While vancomycin has been effective with in vitro testing, it has had a high failure rate clinically10, 13, 14). In addition, third generation cephalosporins are not used because they have no bactericidal effects1, 5). Third generation cephalosporins and vancomycin are recommended as initial empirical antibiotics in bacterial meningitis where penicillin-resistant pneumococcal infections have been documented10, 14). In this case, she had taken the conjugated pneumococcal vaccines and there were no bacteria on the initial CSF gram stain, so we started initial antibiotics treatment with ceftriaxone and amikacin. Then we added vancomycin on the second hospital day, and on the third hospital day we discontinued ceftriaxone and added ampicillin after identifying a listeria strain. Susceptibility results showed that the organism was sensitive to vancomycin, ampicillin, and aminoglycosides.
Even after normal initial neuroimaging of meningoencephalitis caused by listeria, follow up studies are needed during the course of the infection. Listeria infections can be complicated by hydrocephalus and/or brain edema within 1 week of the start of treatment; as was noted in the case presented here. Therefore, repeated neuroimaging is necessary to identify the associated complications1, 7).
In conclusion, we treated an unusual case of meningoencephalitis caused by L. monocytogenes in a healthy child without any underlying disease including an immune disorder. If gram-positive bacilli are observed in the CSF, meningoencephalitis caused by L. monocytogenes should be suspected; repeat CSF studies and neuroimaging are essential for early detection of neurological complications3, 15).



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


