Article Contents
| Korean J Pediatr > Volume 54(5); 2011 |
Abstract
Respiratory syncytial virus (RSV) is a major cause of respiratory infection in children. Most of the pediatric population have RSV infection before the age of 2, and recurrent infections are common even within one season. Chronic lung disease, prematurity, along with congenital heart disease (CHD) are major risk factors in severe lower respiratory infection. In hemo-dynamically significant CHD patients with RSV infection, hospitalization is usually needed and the possibility of treatment in intensive care unit and the use of mechanical ventilator support are known to increase. Therefore the prevention of RSV infection in CHD patients is mandatory. The current standard for RSV prevention is immunoprophylaxis by palivizumab. Immunoprophylaxis is recommended monthly in hemodynamically significant CHD patients, up to 5 months. Motabizumab, a second generation drug and newly developing RSV vaccines are also expected to play a key role in RSV prevention in the future. The prophylaxis of RSV infection in CHD patients is cost-effective in both the medical aspect of the patients as well as the socio-economic aspect. Therefore an effort to promote prevention should be made by not only the family of the patients but also by the government.
Respiratory syncytial virus (RSV) is a common pathogen in causing acute upper respiratory infection in all ages. RSV is a cause of frequent respiratory infection for children in particular. Most of the children are infected at least once by RSV by the age of 2 and symptomatic recurrent infections are also common even within one season1). In children under the age of 2 with high risk factors, severe lower respiratory infection may develop2). Risk factors that may cause fatal outcome after RSV infection includes chronic lung disease, congenital heart disease (CHD), and prematurity3). The indication and method of prevention for RSV infection in children with CHD will be discussed in this article.
Children diagnosed as CHD with RSV infection are known to have severe progress2,3). The study for safety and efficacy of RSV prevention in CHD patients have been rapidly progressing since the development of palivizumab4). Palivizumab (MEDI-493) is a humanized monoclonal antibody to F glycoprotein and has neutralizing and fusion inhibitory effect against RSV5). Studies proving the efficacy of RSV prevention through palivizumab in CHD patients have been developing, and multinational, randomized, double-blind trials have proved its safety and efficacy6). This study included patients who were 1 year old or younger, with hemodynamically significant CHD. Hemodynamically significant CHD was defined as increased pulmonary blood flow with or without cyanosis, pulmonary venous congestion, pulmonary hypertension, and remaining residual hemodynamic abnormality after surgical correction, and these patients were recommended immunoprophylaxis of RSV infection. In a recently published study on RSV infection and hospitalization risk factors in CHD patients, Down syndrome and cardiomyopathy patients with RSV infection were also candidates who needed intensive care as well as the above mentioned patients7). Through studies that have been conducted, the American Academy of Pediatrics (AAP) has included children with bronchopulmonary dysplasia, prematurity, hemodyanamically significant cyanotic and acyanotic CHD patients as high risk group for RSV infection, and expanded the age limit to 2 years of age or younger2). Because hemodynamically significant CHD patients show severe progress, prevention of RSV infection is recommended.
The primary prevention of RSV infection is minimizing exposure to RSV. Even after an exposure, minimizing recurrent exposure can reduce infection. Other preventive measures include the following: 1) Reduce exposure to cigarette smoke 2) Restrict participation of child care in high risk patients during RSV season. 3) Encourage hand washing in all circumstances. Families with high risk patients who have older siblings are especially in need of intensive hand washing8). Because RSV is highly contagious, special caution is needed for infants with CHD who are hospitalized9). Therefore in high risk patients for RSV infection who are hospitalized need intense primary prevention, such as hand washing of medical staff, wearing a surgical mask and eye protection10). These efforts should be made more vigorously and thoroughly during RSV season.
Immunoglobulin for RSV (RSV-IVIG) was developed and first used in 1996 (RespiGam®, MedImmune, LLC, Gaithersburg, MD, USA). RSV-IVIG was first developed for preterm infants and it was contraindicated at first for patients diagnosed as CHD. Through controlled trial, hospitalization due to RSV infection was proven to decrease up to 41-63% after use of RSV-IVIG11). However, there was a possibility that the use of RSV-IVIG in children diagnosed as CHD increased the morbidity and mortality12). Also, because RSV-IVIG is a type of human blood product, there was a possibility of increased blood borne infection and interference of live vaccines such as measles, mumps, and rubella (MMR). Therefore the product was not used since the year 2003.
Palivizumab (Synagis®, MedImmune, LLC, Gaithersburg, MD, USA) was developed by grafting the six complementarity determining regions (CDRs) of mouse MAb to human frameworks5). Palivizumab has received US FDA license in 1998, and it has been used to prevent serious lower respiratory tract infection caused by RSV infection ever since. The safety and efficacy was proven even for high-risk patients since then and currently, it is recognized as the gold standard in RSV prevention. Even in times when RSV-IVIG was being used, palivizumab was preferred because of the easy method of administration (intramuscular injection) and lower interference to MMR and varicella vaccine. Since RSV-IVIG is no longer available, palivizumab is the only licensed product for prevention of RSV infection. Infants diagnosed as CHD are considered as high-risk group for severe lower respiratory infection due to RSV infection, therefore immunoprophylaxis is recommended using palivizumab2,6,7,13-16). Patients with hemodynamically significant cyanotic and acyanotic CHD should start administration of palivizumab before the RSV season starts. Commonly recommended administration dose for palivizumab is 15 mg/kg, intramuscularly once per month for a maximum of five doses2,3). For most of the patients, the compliance under this schedule is tolerable. However, for the few non-compliant patients, home-based program for scheduled palivizumab administration may be helpful in reducing hospitalization caused by RSV infection17). Hemodynamically significant CHD is described in Table 118). In these CHD infants categorized as high-risk group for RSV infection, if RSV prevention is not conducted effectively, mortality after hospitalization is known to increase3). Particularly in these vulnerable infants who are less than 6 months old, the result of RSV infection is more catastrophic. These patients are more prone to hospitalization in the intensive care unit and they have higher possibility of ventilator care19). The blood level of palivizumab after operation under cardiopulmonary bypass may drop by 58%, therefore CHD patients who need continuous prevention for RSV needs to administer palivizumab (15 mg/kg) soon after operation2). Through a randomized controlled study the effectiveness of palivizumab for RSV infection in CHD patients has been proven4). In this study, patients who were administered palivizumab were hospitalized less often (5.3 versus 9.3%), were in need of oxygen administration less often (178 versus 658), and the total of hospitalization days were reduced (367 versus 876). Therefore, the use of palivizumab in prevention of RSV infection in CHD patients can be cost-effective in both the medical aspect of the patients as well as the socio-economic aspect20-22). Although there are many studies reporting the advantages in socio-economic aspects after prophylaxis of RSV infection in CHD patients under the age of 2 years, Korean medical insurance restricts the use of palivizumab to infants of hemodynamically significant CHD patients under the age of 1 year, unfortunately. Further studies and proposals in this matter are imminent. Complications after administration of palivizumab are very rare (<1 per 100,000 recipients), but there was a report of severe hypersensitivity18). However, in a study reporting on the safety of palivizumab, there were no significant complications reported in the 2 years that palivizumab was administered, which may prove that it is safe to use23,24). In some studies, there were reports on RSV infection resistant to palivizumab25). However, there was a report observing 458 infants who were administered with palivizumab without any mutation, which may also prove that palivizumab is effective in RSV infection prophylaxis without much complication26). Recently, the idea that palivizumab can be used not only for prophylaxis but also for treatment has been mentioned. However, further study for treatment effect of palivizumab to RSV in a large population is warranted27,28).
Motavizumab (MEDI-524, Numax®, MedImmune, LLC, Gaithersburg, MD, USA) is a second generation monoclonal antibody for RSV. It is known to be more potent than palivizumab to RSV infection in high-risk patients. Motavizumab has passed the US FDA biologic license application in January of 2008 and is under a large scale clinical study29,30).
Vaccination for RSV infection has not yet been developed. Theoretically, vaccination is a more effective long term preventive method than passive immunization such as palivizumab. Therefore there were numerous efforts trying to develop live attenuated, viral and bacterial vectored, and adjuvant subunit vaccines31-33). However, development of effective RSV vaccines is not fulfilled even under these massive efforts. The interest in developing RSV vaccination is continuously increasing, and recently acquiring immunity of RSV using mucosal and systemic immune response by nanoparticle vaccine is under study34). However, there are many remaining limitations in developing an effective and safe RSV vaccine35,36).
Patients, under the age of 2 years, diagnosed as hemodynamically significant CHD are high-risk group for serious RSV infection. Immunoprophylaxis in these high-risk patients are highly recommended. The proven safe recommended administration dose for immunoprophylaxis of RSV infection is injecting 15mg/kg of palivizumab, intramuscularly once per month for a maximum of five doses. Motavizumab as well as RSV vaccine, currently under development, may contribute to RSV prevention in the future.
Acknowledgment
The authors are grateful to Su-Jin Park, Division of Pediatric Cardiology, Severanse Cardiovascular Hospital, Yonsei University Health System for language consultation.
Notes
The authors don't have any relevant affiliations or financial involvement with any organization related to this topic.
References
1. Groothuis JR, Hoopes JM, Jessie VG. Prevention of serious respiratory syncytial virus-related illness. I: Disease pathogenesis and early attempts at prevention. Adv Ther 2011;28:91–109.



2. Committee on infectious diseases. From the American Academy of Pediatrics: Policy statements-Modified recommendations for use of palivizumab for prevention of respiratory syncytial virus infections. Pediatrics 2009;124:1694–1701.


3. Welliver RC Sr, Checchia PA, Bauman JH, Fernandes AW, Mahadevia PJ, Hall CB. Fatality rates in published reports of RSV hospitalizations among high-risk and otherwise healthy children. Curr Med Res Opin 2010;26:2175–2181.


4. Feltes TF, Cabalka AK, Meissner HC, Piazza FM, Carlin DA, Top FH, et al. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr 2003;143:532–540.


5. Johnson S, Oliver C, Prince GA, Hemming VG, Pfarr DS, Wang SC, et al. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J Infect Dis 1997;176:1215–1224.


6. Tulloh R, Marsh M, Blackburn M, Casey F, Lenney W, Weller P, et al. Recommendations for the use of palivizumab as prophylaxis against respiratory syncytial virus in infants with congenital cardiac disease. Cardiol Young 2003;13:420–423.


7. Kristensen K, Stensballe LG, Bjerre J, Roth D, Fisker N, Kongstad T, et al. Risk factors for respiratory syncytial virus hospitalisation in children with heart disease. Arch Dis Child 2009;94:785–789.


8. Subcommittee on diagnosis and management of bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics 2006;118:1774–1793.


9. Kneyber Mcj EW, Steyerberg R, de Groot HA, Moll HA. Long-term effects of respiratory syncytial virus (RSV) bronchiolitis in infants and young children: a quantitative review. Acta Paediatr 2000;89:654–660.


10. Hall CB, Douglas RG Jr. Nosocomial respiratory syncytial viral infections. Should gowns and masks be used? Am J Dis Child 1981;135:512–515.


11. The PREVENT Study Group. Reduction of respiratory syncytial virus hospitalization among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. Pediatrics 1997;99:93–99.


12. Simoes EA, Sondheimer HM, Top FH, Meissner HC, Welliver RC, Kramer AA, et al. The Cardiac Study Group. Respiratory syncytial virus immune globulin for prophylaxis against respiratory syncytial virus disease in infants and children with congenital heart disease. J Pediatr 1998;133:492–499.


13. Samson L. Prevention of respiratory syncytial virus infection. Paediatr Child Health 2009;14:521–532.



14. Groothuis JR, Hoopes JM, Hemming VG. Prevention of serious respiratory syncytial virus-related illness. II: Immunoprophylaxis. Adv Ther 2011;28:110–125.


15. Weisman LE. Respiratory syncytial virus (RSV) prevention and treatment: past, present, and future. Cardiovasc Hematol Agents Med Chem 2009;7:223–233.


16. Krilov LR. Respiratory syncytial virus disease: update on treatment and prevention. Expert Rev Anti Infect Ther 2011;9:27–32.


17. Frogel MP, Stewart DL, Hoopes M, Fernandes AW, Mahadevia PJ. A systematic review of compliance with palivizumab administration for RSV immunoprophylaxis. J Manag Care Pharm 2010;16:46–58.


18. Meissner HC, Long S. Revised indications for the use of palivizumab and respiratory syncytial virus immune globulin intravenous for the prevention of respiratory syncytial virus infections. Pediatrics 2003;112:1447–1452.


19. Fitzgerald DA. Preventing RSV bronchiolitis in vulnerable infants: the role of palivizumab. Paediatr Respir Rev 2009;10:143–147.


20. Smart KA, Lanctt KL, Paes BA. The cost effectiveness of palivizumab: a systematic review of the evidence. J Med Econ 2010;13:453–463.


21. Prescott WA, Doloresco F, Brown J, Paladino JA. Cost effectiveness of respiratory syncytial virus prophylaxis: a critical and systematic review. Pharmacoeconomics 2010;28:279–293.


22. Hampp C, Kauf TL, Saidi AS, Winterstein AG. Cost-effectiveness of respiratory syncytial virus prophylaxis in various indications. Arch Pediatr Adolesc Med 2011;5 05 [Epub ahead of print].
23. Lacaze-Masmonteil T, Seidenberg J, Mitchell I, Cossey V, Cihar M, Csader M, et al. Evaluation of the safety of palivizumab in the second season of exposure in young children at risk for severe respiratory syncytial virus infection. Drug Saf 2003;26:283–291.


24. Null D, Pollara B, Dennehy PH, Steichen J, Snchez PJ, Givner LB, et al. Safety and immunogenicity of palivizumab (Synagis) administered for two seasons. Pediatr Infect Dis J 2005;24:1021–1023.


25. Adams O, Bonzel L, Kovacevic A, Mayatepek E, Hoehn T, Vogel M. Palivizumab-resistant human respiratory syncytial virus infection in infancy. Clin Infect Dis 2010;51:185–188.


26. DeVincenzo JP, Hall CB, Kimberlin DW, Snchez PJ, Rodriguez WJ, Jantausch BA, et al. Surveillance of clinical isolates of respiratory syncytial virus for palivizumab (Synagis)-resistant mutants. J Infect Dis 2004;190:975–978.


28. Hu J, Robinson JL. Treatment of respiratory syncytial virus with palivizumab: a systematic review. World J Pediatr 2010;6:296–300.


30. Gill MA, Welliver RC. Motavizumab for the prevention of respiratory syncytial virus infection in infants. Expert Opin Biol Ther 2009;9:1335–1345.


31. Fulginiti VA, Eller JJ, Sieber OF, Joyner JW, Minamitani M, Meiklejohn G. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am J Epidemiol 1969;89:435–448.


32. Murphy BR, Walsh EE. Formalin-inactivated respiratory syncytial virus vaccine induces antibodies to the fusion glycoprotein that are deficient in fusion-inhibiting activity. J Clin Microbiol 1988;26:1595–1597.



33. Olson MR, Varga SM. CD8 T cells inhibit respiratory syncytial virus (RSV) vaccine-enhanced disease. J Immunol 2007;179:5415–5424.


Table 1
Respiratory Ryncytial Virus Immunoprophylaxis for Infants and Young Children with Congenital Heart Disease
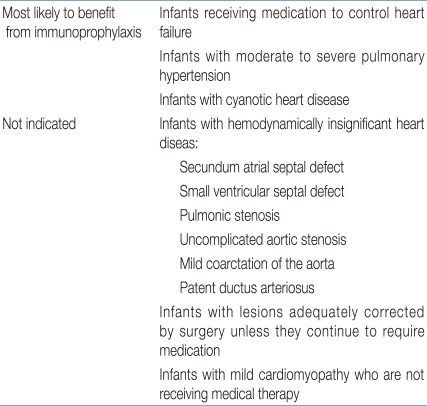
Adapted from: revised indications for the use of palivizumab and respiratory syncytial virus immune globulin intravenous for the prevention of respiratory syncytial virus infections. Pediatrics 2003;112:144218).



 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


