< Previous Next >
Article Contents
| Korean J Pediatr > Volume 60(2); 2017 |
|
Abstract
Purpose
The aims of this study were to evaluate the safety and pharmacokinetics of levetiracetam (LEV) in neonates with seizures and to establish a population pharmacokinetics (PPK) model by using the software NONMEM.
Methods
A retrospective analysis of 18 neonatal patients with seizures, who were treated with LEV, including 151 serum samples, was performed. The mean loading dose was 20 mg/kg, followed by a mean maintenance dose of 29 mg/kg/day.
Results
Seventeen neonates (94%) had seizure cessation within 1 week and 16 (84%) remained seizure-free at 30 days under the LEV therapy. The mean serum concentration of LEV was 8.7 µg/mL. Eight samples (5%) were found above the therapeutic range. No serious adverse effects were detected. In the PPK analysis for Korean neonates, the half-life was 9.6 hours; clearance, 0.357 L/hr; and volume of distribution, 4.947 L, showing differences from those in adults.
Neonatal seizures may adversely impact neurodevelopmental outcome, so their prompt recognition and treatment is important1,2). Phenobarbital and phenytoin are the most commonly administered antiepileptic medications for neonatal seizures. However, there are increasing concerns over the long time adverse effects since they were shown to increase neuronal apoptosis in animal models and induce cognitive impairment in infants and toddlers3).
Levetiracetam (LEV) is a new antiepileptic drug (AED), which is mainly used for the adjunctive treatment of partial seizures, approved in children and adults4,5,6). LEV showed the efficacy and safety to children7). There have been no reports of a significant risk for severe adverse effects, but little evidence for the neonates were known. Furthermore, studies on animals have shown that LEV does not cause neuronal apoptosis in the immature brain and shows promise as a neuroprotective agent8,9).
LEV has been used increasingly in the treatment of neonates with seizures in Korea; however, the data about the efficacy and safety of LEV in neonates are insufficient, and pharmacokinetic parameters in Korean neonates are unknown. This study analyzes the safety and pharmacokinetics of using LEV in neonates with seizures and develops a population pharmacokinetics (PPK) model of LEV in Korean neonates.
Eighteen neonates with electro-clinical or electrographic-only confirmed seizures who received intravenous and oral LEV in the 2 hospital neonatal intensive care units of Yonsei University College of Medicine during a 2-year period (June 2013 to June 2015) were included. The major etiologies for neonatal seizures were hypoxic-ischemic encephalopathy (Table 1). The postmenstrual age, postnatal age, and the bodyweight at the time of first LEV administration was 47.6 weeks, 12.6 weeks, and 4,270 g, respectively.
This study was a retrospective analysis of LEV as a monotherapy or add-on treatment for neonatal seizures. They received initial intravenous doses of 10–20 mg/kg and gradually increased to 20–60 mg/kg according to their clinical status. For each patient, seizure type, frequency, coadministered medications, and adverse events were collected in as much detail as possible. Routine electroencephalograms (EEGs) and laboratory tests were assessed over 3 months. The study protocol was approved by the Institutional Review Board.
Actual sampling times and dosing times were recorded by a nurse. The sampling times to last LEV intake were generally between 6 and 23 hours, with 1 sample after the dose. Serum LEV concentrations were assayed using a chromatography-mass spectrometry (QTRAP 5500 LC-MS/MS System, Framingham, MA, USA). The working assay range was 1–100 µg/mL.
A compartment model was used to build a population pharmacokinetic model, with an exponential error model for random inter-individual variability distributed as a normal distribution with mean zero and variance ω2. Covariate selection was performed using stepwise covariate modeling with likelihood ratio test at significance levels of P<0.01 for forward selection and P<0.001 for backward elimination. The covariates tested included gender, birth weight, current weight, gestational age, postnatal age, postmenstrual age, Apgar score, clearance maturation, and serum creatinine concentration. Dose and coadministered medications were additionally tested for any significant influence.
All analyses were performed using NONMEM ver. 7.3 (ICON Development Solutions, Ellicott City, MD, USA) with the first-order conditional estimation method with interaction and PsN tool (version 3.4.2) was used for covariate modeling. The plots were created using R (http://www.r-project.org). The developed model was evaluated by comparing the predicted serum LEV concentration versus time curve with the observation.
Mean gestational age was 35.0 weeks and mean birth weight was 2,393 g (Table 1). Among 151 samples, the mean sampling time after medication was 11.7 hours. The mean initial dose of LEV was 20.0±16.1 mg/kg, and the mean daily maintenance dose was 29.0±20.1 (Table 2). The main indication for the use of LEV was continuation of seizures despite management with phenobarbital or phenytoin. All patients except 1 neonate were receiving concomitant AEDs. The AED included LEV (5.5%), phenobarbital with LEV (61.1%), and phenobarbital, phenytoin with LEV (33.3%).
The response to treatment was based on clinical data and the results of routine EEG monitoring. One hundred percent seizure cessation was achieved by 72 hours after loading doses. The results showed that 94% of patients had seizure cessation within the first week, and 89% remained seizure-free under the LEV therapy until 1 month. In addition, EEGs were markedly improved in 60% patients at 2 weeks and 50% patients at 1 month (Table 3).
LEV plasma levels were in the range of 1–41 µg/mL (reference values, 6–20 µg/mL), and 83 of 151 samples (55%) were included in the therapeutic range. Only 8 samples (5%) were found above the therapeutic range. No immediate adverse effects were noted, and the laboratory results at the time of LEV measurement were within normal (Table 4).
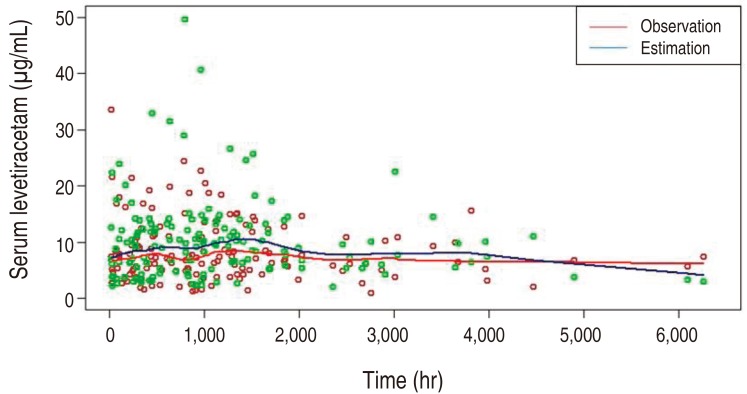
The pharmacokinetics of LEV was best described by a one-compartment model, parameterized by the volume of distribution (V) and clearance (CL). Here, the drug absorption kinetics was not considered in the model as the sampling times between 6 and 23 hours were not early enough to model the absorption phase. The typical parameter estimates (relative standard error%) for the selected model were 1.15 L/kg (29.7%) and 0.083 L/hr/kg (12.7%) for V and CL, respectively, yielding 4.947 L for V and 0.357 L/hr for CL when evaluated at the median weight of 4.3 kg and the median postmenstrual age of 48.7 weeks. The interindividual variabilities were shown (Table 5). Covariate modeling revealed that, around its typical value, CL increases parabolically with clearance maturation and decreases exponentially with serum creatinine concentration. When the predicted serum LEV concentration was compared with the observed concentration over time, overall the prediction matched with the observation, supporting the validity of the model developed (Fig. 1).
The results of this study demonstrate the safety and pharmacokinetics of LEV in neonates with neonatal seizures. This is the first study that developed a PPK model for LEV in Korean neonates.
Neonatal seizures are common, with an incidence of 1.5–3.5/1,000 in term newborns and 10–130/1,000 in preterm newborns10). Neonatal seizures are usually caused by severe cerebral pathology and have long-term consequences on the developing brain. Despite their frequency and clinical significance, currently, there are lack of randomized controlled trials to validate a treatment procedure for neonatal seizures11). Available data indicate that phenobarbital or phenytoin remains the first-line treatment for neonatal seizures12). However, phenobarbital and phenytoin relieved seizures in only 43% and 45% of neonates, respectively13). On the other hand, there is concern regarding long time adverse effects of traditional AEDs since they were shown to trigger apoptotic neurodegeneration in vitro14) and to induce cognitive impairment in infants and toddlers15). Thus, there is increasing interest in new AEDs as safer therapeutic options for neonatal seizures.
LEV is an effective AED licensed as an adjunctive therapy in seithe treatment of partial seizures in adults, children, and infants16). In contrast to traditional AEDs, LEV does not increase neuroapoptosis and shows promise as a neuroprotective agent in animal models. Furthermore, recent studies in children and infants including neonates revealed ideal pharmacokinetics and favorable clinical and safety profiles for LEV. There have been no reports of a severe, life threatening adverse effects. The most commonly observed side effects were somnolence, headache, and behavioral problems17). However, due to the lack of experience in neonate, further research including pharmacokinetic and pharmacodynamic analysis should be needed.
Several pediatric studies have reported marked improvement in neonatal seizure frequency with the use of LEV. A prospective study as a first-line therapy in 38 neonates, including premature infants, showed 78% of neonates were seizure-free at the end of the first week. LEV was tolerated extremely well with somnolence during titration, without no serious adverse effects18).
Abend et al.7) showed the safety and effect of LEV in 23 neonates received an initial bolus dose of 10–20 mg/kg with up to a mean maximum daily dosage of 45 mg/kg/day. In another retrospective study as a second-line therapy, 12 preterm infants received an initial loading dose of 50 mg/kg and maintenance doses of 25 mg/kg/dose, showed complete cessation at 72 hours19). No immediate side effects were noted. But due to the small sample size, variable dosing, and no pharmacokinetic analysis, further study should be needed.
In Korea, LEV has been used in the treatment of children with epilepsy since 2007. A study in adults showed a 44% reduction in seizures over the 16-week treatment period20). For the patients between 1 month and 4 years of age receiving a median starting dose of 13 mg/kg and a maintenance dose of 52 mg/kg/day, 33% of patients experienced a more than 50% seizure reduction, and 13% of patients became seizure-free21). No serious side effects were reported. But in Korean neonates including preterm infants, there is no reported data for the safety and efficacy of LEV.
The average dosage of LEV in children and adults is 20 mg/kg/day initially, to be increased up to 30–60 mg/kg/day, and trough concentrations are typically in the range of 6–20 µg/mL. However, there is a lack of data on the pharmacokinetics in the neonatal age group, and reabsorption of orally administered LEV can be postulated to be limited in sick neonates. In view of the lack of this data, we decided on relatively low LEV doses, which were further increased according to the clinical conditions. In this study, an initial loading dose of 20-mg/kg LEV was used, followed by a median dose of 29 mg/kg/day for maintenance therapy. Serum levels of LEV were in the therapeutic range in 83 samples (55%), including when changing administration from intravenous to oral.
LEV is rapidly and completely absorbed, quickly reaches peak and steady state within 1.5 hours, and exhibits linear time-dependent kinetics. Its major elimination route is through the kidneys. It is negligibly metabolized by cytochrome P450 liver enzymes, resulting in low drug interactions with AEDs or other drugs22). Neonates were found to have a higher clearance rate (neonates 1.21 mL/min/kg, vs. adults 0.96 mL/min/kg), a higher volume of distribution (0.89 L/kg vs. 0.5–0.7 L/kg), and a longer half-life (8.9 hours vs. 6–8 hours). This is because neonates have decreased glomerular filtration and higher total body water content than children and adults23). In this study, similar results of pharmacokinetics were shown.
PPK model of LEV in Chinese children aged 0.5–14 years with epilepsy showed body weight was a significant covariate for LEV clearance24). Sharpe and Haas9) noted the clearance in the neonates was modeled as a simple function of postnatal age. In Merhar et al.23)'s study of neonates, pharmacokinetic analysis pointed to creatinine clearance as a crucial factor for LEV clearance. The estimated value of clearance in our model showed postmenstural age and creatinine to be the influential factors in LEV clearance. There was no statistically significant association between CL/F or V/F and concomitant use of AEDs.
Our study has limitations: small sample size, no sampling in absorption phase with only one sample after dosing, and short-term follow-up duration. In addition, the LEV dosing regimen was not standardized because there have been too few studies evaluating the optimal dose with neonatal pharmacokinetic data. Most cases received LEV as adjuvant therapy, so it is difficult to determine its benefit as a monotherapy based on these data. More standardized, larger, and long-term study is required.
Nevertheless, the pharmacokinetic parameters and research in Korean neonates are not known, and this study provides significant evidence that LEV is an effective and safe anticonvulsant in the neonate population, including in premature infants. LEV should be considered a reasonable alternative to phenobarbital to control neonatal seizures.
Notes
Conflict of interest:
No potential conflict of interest relevant to this article was reported.
References
1. Glass HC, Pham TN, Danielsen B, Towner D, Glidden D, Wu YW. Antenatal and intrapartum risk factors for seizures in term newborns: a population-based study, California 1998-2002. J Pediatr 2009;154:24–28.e1.


2. Kirmani BF. Levetiracetam: an alternative option in preterm neonates for acute seizure management. Mol Cell Epilepsy 2014;1:e19
3. Mruk AL, Garlitz KL, Leung NR. Levetiracetam in neonatal seithezures: a review. J Pediatr Pharmacol Ther 2015;20:76–89.


5. Shorvon SD, Löwenthal A, Janz D, Bielen E, Loiseau P. Multicenter double-blind, randomized, placebo-controlled trial of levetiracetam as add-on therapy in patients with refractory partial seizures. European Levetiracetam Study Group. Epilepsia 2000;41:1179–1186.


7. Abend NS, Gutierrez-Colina AM, Monk HM, Dlugos DJ, Clancy RR. Levetiracetam for treatment of neonatal seizures. J Child Neurol 2011;26:465–470.



8. Manthey D, Asimiadou S, Stefovska V, Kaindl AM, Fassbender J, Ikonomidou C, et al. Sulthiame but not levetiracetam exerts neurotoxic effect in the developing rat brain. Exp Neurol 2005;193:497–503.


9. Sharpe C, Haas RH. Levetiracetam in the treatment of neonatal seizures. J Pediatr Neurol 2009;7:79–83.
10. Volpe JJ. Neurology of the newborn. Philadelphia (PA): Elsevier Health Sciences, 2008.
11. World Health Organization. Guidelines on neonatal seizures. Geneva: World Health Organization, 2011.
12. Bartha AI, Shen J, Katz KH, Mischel RE, Yap KR, Ivacko JA, et al. Neonatal seizures: multicenter variability in current treatment practices. Pediatr Neurol 2007;37:85–90.


13. Painter MJ, Scher MS, Stein AD, Armatti S, Wang Z, Gardiner JC, et al. Phenobarbital compared with phenytoin for the treatment of neonatal seizures. N Engl J Med 1999;341:485–489.


14. Bittigau P, Sifringer M, Ikonomidou C. Antiepileptic drugs and apoptosis in the developing brain. Ann N Y Acad Sci 2003;993:103–114.


15. Camfield CS, Chaplin S, Doyle AB, Shapiro SH, Cummings C, Camfield PR. Side effects of phenobarbital in toddlers; behavioral and cognitive aspects. J Pediatr 1979;95:361–365.


16. Glauser TA, Ayala R, Elterman RD, Mitchell WG, Van Orman CB, Gauer LJ, et al. Double-blind placebo-controlled trial of adjunctive levetiracetam in pediatric partial seizures. Neurology 2006;66:1654–1660.


17. Opp J, Tuxhorn I, May T, Kluger G, Wiemer-Kruel A, Kurlemann G, et al. Levetiracetam in children with refractory epilepsy: a multicenter open label study in Germany. Seizure 2005;14:476–484.


18. Ramantani G, Ikonomidou C, Walter B, Rating D, Dinger J. Levetiracetam: safety and efficacy in neonatal seizures. Eur J Paediatr Neurol 2011;15:1–7.


19. Khan O, Cipriani C, Wright C, Crisp E, Kirmani B. Role of intravenous levetiracetam for acute seizure management in preterm neonates. Pediatr Neurol 2013;49:340–343.


20. Heo K, Lee BI, Yi SD, Huh K, Kim JM, Lee SA, et al. Efficacy and safety of levetiracetam as adjunctive treatment of refractory partial seizures in a multicentre open-label single-arm trial in Korean patients. Seizure 2007;16:402–409.


21. Kim SH, Lim BC, Cho A, Chae JH, Hwang YS, Kim KJ. Efficacy and safety of levetiracetam in children younger than 4 years with intractable epilepsy. J Korean Epilepsy Soc 2009;13:3–7.
Fig. 1
Serum levetiracetam concentrations at each time point, showing that the overall estimation matched well with the observation.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


