To the editor,
School closure during the coronavirus disease 2019 (COVID-19) pandemic reduced physical activity and aggravated obesity in children [
1]. Obesity is a major risk factor for metabolic syndrome (MS) [
2]. Obesity-related asthma is characterized by more severe asthma phenotype [
3,
4]. Previous studies have demonstrated that childhood obesity is not only associated with increased risk for incidental asthma, asthma severity, and morbidity, but also has significant effects on lung function [
5,
6]. A rapid increase in obesity during the COVID-19 pandemic highlights the urgency for exploring MS particularly in asthmatic children. It is important to examine how MS impacts on pulmonary function in asthmatic children. We performed a cross-sectional study to investigate possible associations between pulmonary dysfunction and MS components during the COVID-19 pandemic.
Asthmatic children who attended the Allergy Clinic of Korea University Anam Hospital, Seoul, between January and December of 2021 were participated in this study. Asthma was diagnosed based on the patient's medical history, including recurrent symptoms, the correlation between allergens or triggers and symptom development, response to treatment, presence of other allergic conditions, and family history.Additionally,the diagnosis considered findings from the physical examination, such as wheezing, tachypnea, and nasal flaring, as well as results from laboratory tests, including complete blood count, eosinophil count, and serum total and specific IgE levels. In vivo test results, such as the skin prick test, pulmonary function test, and bronchial provocation test, were also taken into account. MS was diagnosed based on The International Diabetes Federation's definition, which includes waist circumference (WC), triglycerides, high-density lipoprotein cholesterol (HDL-C), blood pressure, and fasting glucose [
7]. Data from 61 subjects were evaluated. The study protocol was approved by the Institutional Review Board of the Korea University Anam Hospital(No.AN16013-001).Anthropometric data as well as results of blood test, pulmonary function test, methacholine bronchoprovocation test, and skin prick test were analyzed. The blood tests, including cholesterol, were conducted in the morning after a minimum of eight hours of fasting.
Table 1 shows how different metabolic components of MS and pulmonary function parameters between asthmatics with and without MS. Among the lipid profiles, the mean triglyceride level was significantly higher in asthmatics with MS than in those without(168.8±124.8 mg/dL vs. 106.5±56.2 mg/dL,
P=0.019). The mean low-density lipoprotein cholesterol(LDL-C)levels were slightly higher in asthmatics with MS than in those without (107.6±22.3 mg/dL vs. 94.8±22.1 mg/dL,
P=0.054) whereas the mean HDL-C levels were higher in asthmatics without MS (53.4±12.4 mg/dL vs. 43.0±7.2 mg/dL,
P=0.002). The mean forced expiratory volume in 1 second (FEV
1) and forced vital capacity (FVC) levels were significantly higher in the asthmatics with MS than in those without (90.7±14.3%pred vs. 82.6±11.7%pred,
P<0.001 and 103.1±19.0%pred vs. 87.2±14.8%pred,
P<0.001, respectively); however, FEV
1/FVC ratio was significantly lower in the asthmatics with MS than in those without (79.7±9.01% vs 84.6±7.67%,
P=0.030). Multivariable linear regression analyses were performed with adjustment for age, sex, and height. FEV
1/FVC ratio showed significant inverse associations with WC (
β=-0.246,
P=0.045) and serum total cholesterol (
β=-0.319,
P=0.020) and LDL-C (
β=-0.317,
P=0.032) but not with body mass index (
β=-0.146,
P=0.256) or triglyceride (
β=-0.066,
P=0.134)(
Table 2).
Obesity is associated with the development and/or worsening of asthma [
5,
8]. Obesity-related asthma is driven by increased sedentary life styles and unhealthy eating habits that are most prevalent in low socioeconomic populations [
3,
4]. Increased childhood overweight and obesity after social distancing and school closure due to the COVID-19 pandemic are serious concerns [
9]. After school closure, children are restricted to staying at home and further reducing opportunities for physical activities and exercise [
9]. A previous observational study of obesity reported that the overweight and obesity rate was 23.9% during Korean children in pre-COVID-19 period; however, this proportion increased to 31.4% during the COVID-19 pandemic [
10].
Since both obesity and asthma are chronic inflammatory diseases, it is possible that pathophysiological interactions exist between the two. Possible explanations for this relationship are adverse effects of dietary cholesterol on airway inflammation and promotion of Th2 inflammation by hypercholesterolemia [
3,
4,
11]. Metabolic derangement may also lead to airway dysfunction via epithelial damage and airway smooth muscle proliferation. MS has also been significantly associated with lung function impairment in obese population, with abdominal obesity being the key determinant of this association [
3,
4,
11].
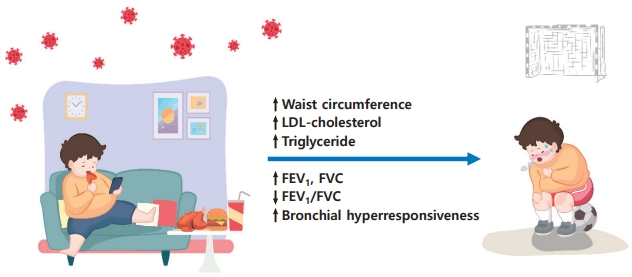
We observed higher FEV
1 and FVC levels but lower FEV
1/FVC ratio in asthmatic children with MS than in those without. Due to early somatic growth occurring in obese children, the lungs grow at an accelerated pace resulting in airway dysanapsis, measured as an FVC increased disproportionately compared to FEV
1 [
10]. Altered pulmonary mechanics in obese asthmatics differ from those observed in classic childhood allergic asthma and likely contribute to more symptoms and poorer asthma control, which are associated with lower asthma-related quality of life and increased health care utilization [
3-
5]. It means that obese asthmatic children are prone to be more symptomatic when they have an attack (
Fig. 1). Thus, obesity and/or MS in children with asthma should be managed more meticulously early in the life.
In the present study, high LDL-C and WC were significantly associated with obstructive pulmonary dysfunction (low FEV1/FVC) in asthmatic children. Measuring WC could provide valuable information when assessing the degree of lung function impairment related to obesity. Although there were significant differences in triglyceride and HDL-C levels between asthmatics with and without MS, we could not find any significant associations with low lung function. However, close observation of impaired lung function according to changes in these parameters is needed.
This study has some limitations.First,it has a small sample size and enrollment. Further observational studies are needed to confirm our results. Secondly, our cross-sectional study design could not assess the causal relationships between MS components and impaired lung function. Therefore, it is not clear how long-term obesity affects pulmonary dysfunction. The present cross-sectional study highlights the effects of obesity on lung function in asthmatic children during the COVID-19 pandemic. Asthmatic children with MS, particularly with increased WC and higher LDL-C level, have pulmonary dysfunction. Together, reduced physical activity during the COVID-19 pandemic aggravates obesity in school-aged children and negatively affects lung function in asthmatic children. Increased awareness of the risk of abdominal obesity in asthmatic children should be addressed to prevent pulmonary dysfunction which may persist into adulthood.
In conclusion, the present study contributes to identifying the association between MS components and pulmonary dysfunction deficits in asthmatic children. It has important implications for public health, indicating that more emphasis should be placed on the development of prevention strategies for MS in asthmatic children.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


