Introduction
Fanconi anemia (FA) is a rare autosomal and X-linked recessive disorder, characterized by physical abnormalities, progressive bone marrow failure (BMF), hypersensitivity to DNA cross-linking agents and predisposition to malignancy
1). It was first described in 1927 by Fanconi
2), who described a family in which three children had pancytopenia and birth defects, such as short stature, hypogonadism and skin disorder. Since then, 1,075 patients were registered in the International Fanconi Anemia Registry (IFAR) between May 1982 and August 2008
3).
FA is considered the most common inherited cause of BMF, which is usually apparent between 6 and 8 years of age. Most patients with FA have birth defects including skin hyperpigmentation and/or cafe au lait spots, skeletal malformations, short stature and urogenital abnormalities
3). However, one-third of the patients have few or none of these features, which makes it difficult to diagnose
4). FA patients may develop myelodysplastic syndrome (MDS), or acute myeloid leukemia (AML), with relative risk of developing AML being 800-fold higher than that of the general population. There is also a strong predisposition to specific solid tumors, including head, neck, gynecological squamous cell carcinoma (SCC) and liver tumors
4,
5).
Hematopoietic stem cell transplantation (SCT) is currently the only treatment to restore normal hematopoiesis because of nonresponse to immunosuppressive therapy, such as antilymphocyte globulin, antithymocyte globulin (ATG) and cyclosporine, which is usually given to treat idiopathic aplastic anemia (AA)
6). Moreover, because of hypersensitivity to chemotherapeutic agents or radiation, higher risk of developing malignancies, and potential recurrence in the siblings, the approach to SCT should be different from that of idiopathic AA in terms of the donor selection, optimal timing of SCT, and the choice of conditioning regimen. Also, the prognosis after SCT is quite different
7,
8). Therefore, FA should be sought carefully in any patients with AA or BMF syndromes in children and young adults.
A screening study with DNA cross-linking agents in patients with BMF and clinical manifestation of 6 FA patients was published from our institution in 1997 and 1998, respectively
9,
10). The present study represents an update and long-term follow-up study of 12 patients diagnosed with FA at the Chonnam National University Hospital over the last 20 years. Clinical presentations, laboratory findings, diagnostic methods, treatment modalities, outcomes and long-term sequelae of patients were retrospectively analyzed to increase awareness of this rare but very challenging genetic disease.
Discussion
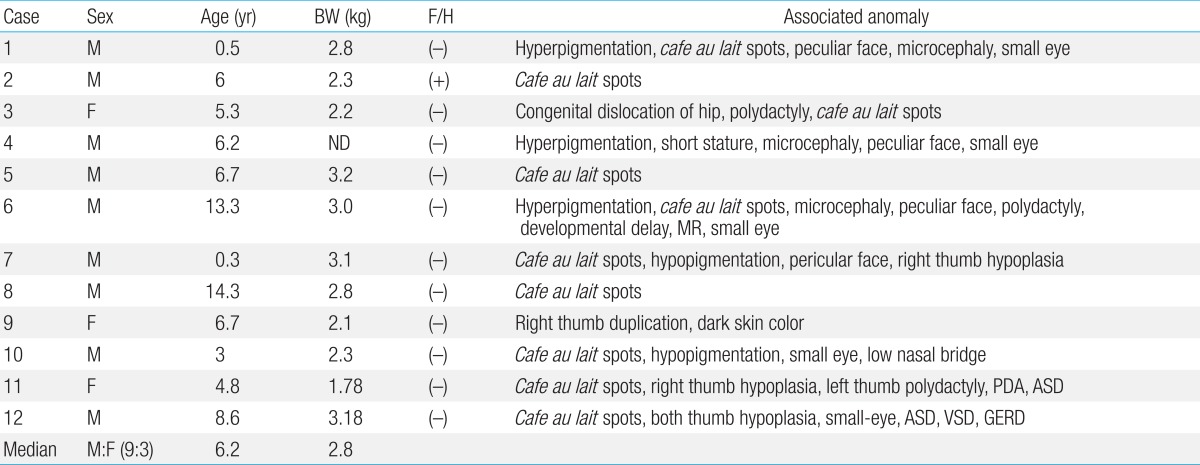
FA is a genetic disorder characterized by multiple congenital anomalies, hematological abnormalities and predisposition to a variety of cancers. Approximately two-thirds of FA patients present with a combination of various congenital abnormalities such as short stature, Fanconi facies with microophthalmia, skeletal deformities, skin hyperpigmentation, and cardiac, renal, genitourinary, and/or other malformations
3,
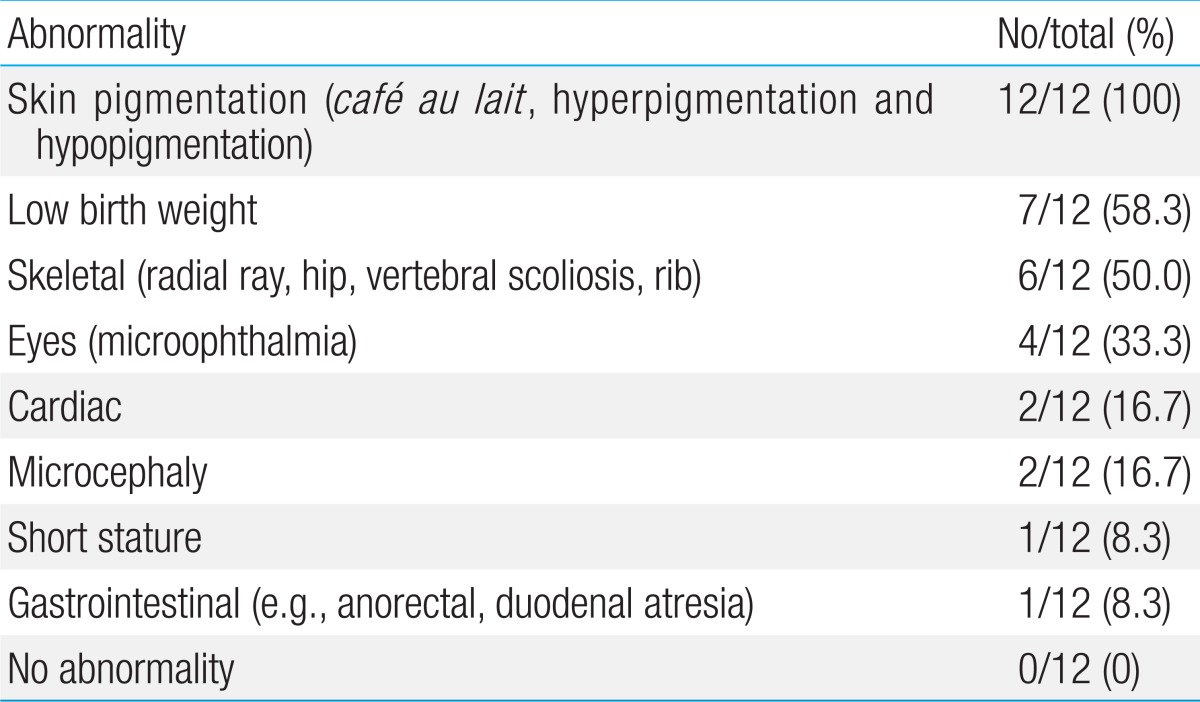
17). Skin abnormalities have been described in the literature in 55% of cases of FA
4). However, all patients in this study had skin hyperpigmentation and/or
cafe-au-lait spots. Skeletal abnormalities, found in 43% of cases of FA, were hypoplasia or absence of the thumbs, bifid or supernumerary thumbs, and hypoplasia or absence of the radius
4). One third in this study had skeletal abnormalities. Other less common abnormalities were 2 cardiac anomalies and 1 gastrointestinal defect. Physical abnormalities can be subtle or absent as 3 patients (cases 2, 5, and 8) in the present study showed cafe-au-lait spots only.
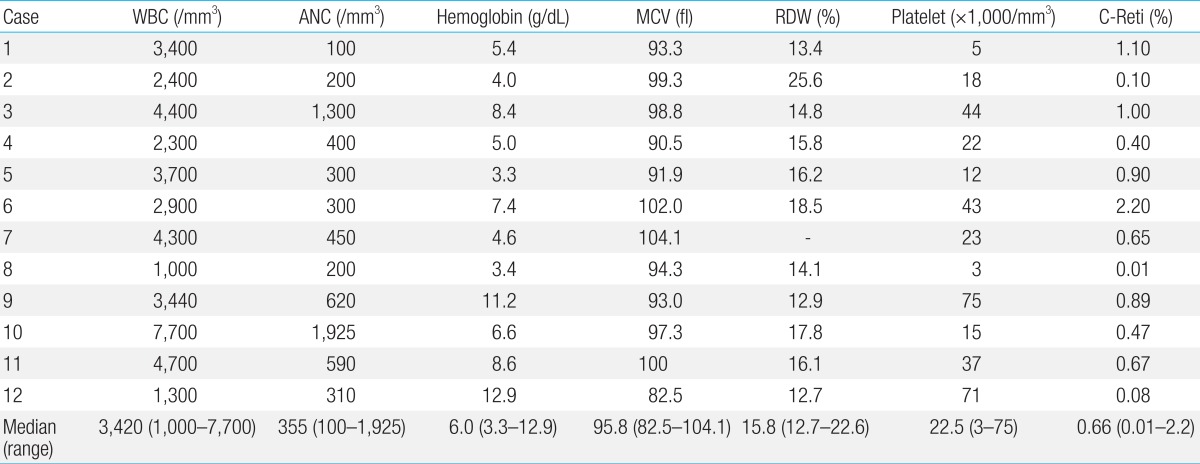
Hematologic abnormalities are the most important clinical features of FA. BMF develops at a median age of 7 years (range, birth to 41 years)
5,
18). For the majority of patients, the suspicion of FA is made only after the onset of pancytopenia. Initial hematologic findings are diverse. At birth, blood count is usually normal and macrocytosis is commonly the first detected abnormality followed by thrombocytopenia and anemia. During the first decade of life, about 75%-90% of FA patients develop BMF
18).
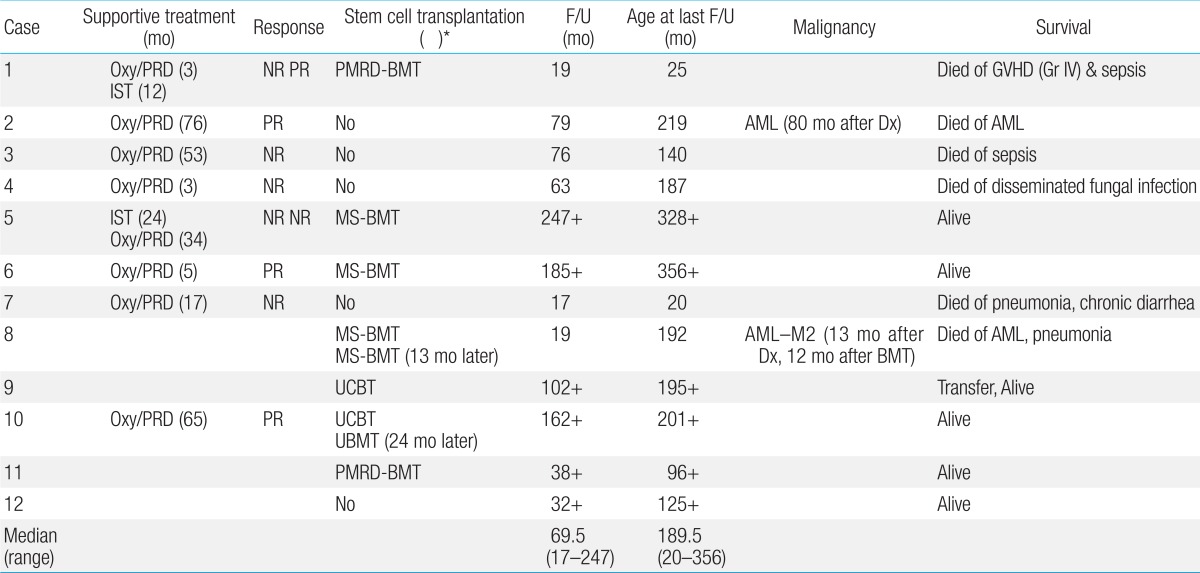
In some patients, the underlying diagnosis of FA is not known until MDS or AML occurs. The relative risk of developing AML is approximately 800 fold higher than that of the general population, with the median onset of 14 years
19). In this study, 2 patients (cases 2 and 8) developed AML at 15.4 and 18.1 years of age, respectively. MDS was detected in 23 cases among 119 FA patients cohort at a median of 12 years (range, 2 to 44 years) in a recent study
20). The presentation of MDS at the age of 3 months (case 3) in this study should be extremely rare
15).
In addition to hematologic malignancy, solid tumors develop at markedly higher rates in FA patients. Most of the solid tumors in FA patients are SCC, particularly of the head and neck, esophagus and anogenital regions. Hepatic tumors may also be found at higher rates, which may be related to androgen use. Approximately one-third of FA patients were known to develop a solid tumor by the fourth decade of life
21). However, not a single case of solid tumor has thus far been identified in this study. With wider application of SCT and improved survival, long-term survivors will have a particularly high risk of developing solid tumors.
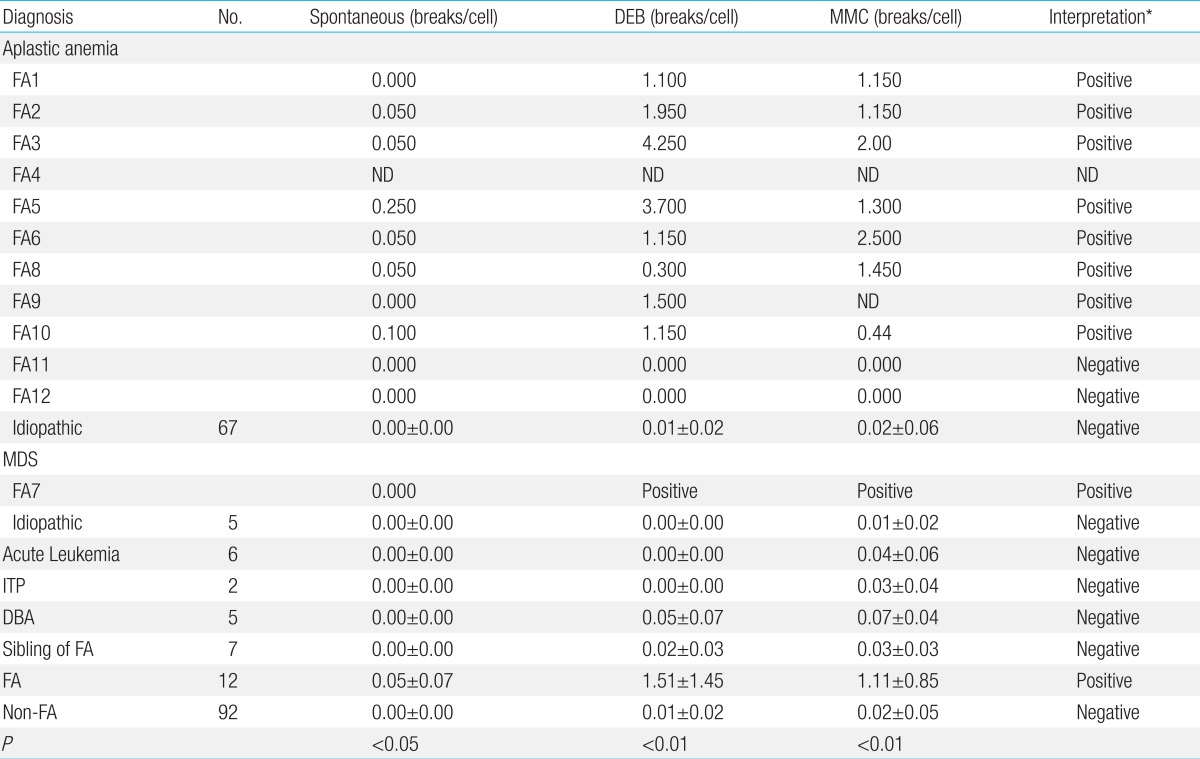
Because of the heterogeneity on clinical features and recessive mode of inheritance FA can not be diagnosed with certainty by careful history and physical examination in patients with BMF. The biologic diagnosis of FA is primarily based on the exquisite sensitivity of FA cells to the cytotoxic and clastogenic effect of DNA cross-linking agents such as DEB or MMC. The chromosomal breakage test with these agents is the technique of reference for diagnosing FA
3,
10,
11). The incidence of FA has been reported to represent 25% to 30% of pediatric AA. In Korea, however, the frequency of FA among patients with AA has not been systematically evaluated and has probably been underestimated as DEB and MMC tests have not been routinely incorporated in the evaluation of patients with AA in every institution. In accordance with the previous study
10), the chromosomal breakage of FA cells to DEB and MMC was distinguishable from non-FA group in this study. Spontaneous chromosomal breakage was not effective in distinguishing FA from non-FA patients because of overlap of breakage ranges
11). In the current study, 5 FA patients did not show spontaneous chromosomal breakages.
Other blood tests such as cell-cycle analysis and evaluation of FANCD2 mono-ubiquitination may also be used to diagnose FA. However, all of these tests can be ambiguous or even falsely negative in patients who develop somatic mosaicism and hematopoietic reversion
22). Because these phenomena have not been demonstrated in fibroblasts, primary fibroblast cells can be tested for chromosomal breakage tests or other tests for FA diagnosis
23). In the current study, 2 patients (cases 11 and 12) with peculiar physical characteristics along with BMF showed negative results for chromosomal breakage tests on blood. Attempts on skin fibroblasts were unfortunately not successful in case 11 and negative in case 12. The possibility of somatic mosaicism or hematopoietic reversion should be contemplated in those cases.
Since the first FA gene was demonstrated in 1992
24), 16 FA complementation groups (FANCs) have been identified (FANC-A, -B, -C, -D1, -D2, -E, -F, -G, -I, -J, -L, -M, -N, -O, -P, and -Q) so far. The majority of FA genes are located on autosomes except FANCB, which is on the X chromosome. Among the 15 complementation groups, FA-A (60%-70%), FA-C (up to 14%), and FA-G (up to 10%) collectively accounts for more than 90% of the FA groups in the western population
17). However, considerable variation in the frequency of each complementation group has been reported according to ethnicity. FA-A and FA-G mutations were detected in 70%-80% and 10%-22% in Japanese population
25). A recent study from Korea identified 6 FA-A (46%), 7 FA-G (54%) and no FA-C among 13 FA patients using MLPA and direct sequencing
26). Unfortunately, 1 patient (case 12) who underwent gene analysis was not able to be subtyped in the present study.
It is crucial to identify patients with FA in the management of BMF patients. Patients with BMF who happen to have underlying undiagnosed FA will not respond to the immunosuppressive therapy that is used to treat patients with idopathic AA. Moreover, patients with FA will often die of toxicity due to hypersensitivity to chemoradiotherapy when given conventional conditioning for SCT, and therefore reduced intensity conditioning regimens should be used for FA patients
8). Also, human leukocyte antigen (HLA)-matched sibling donors should also be screened for FA as the recurrence rate for FA in a sibling is 25% for autosomal recessive disease.
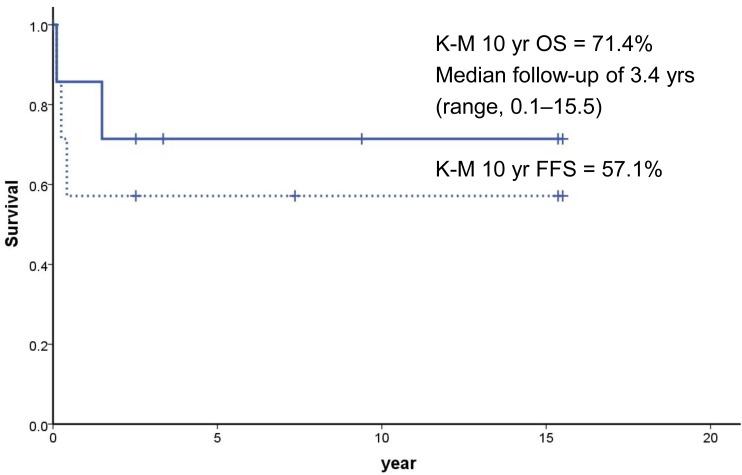
In FA patients, hematological complications are the most life-threatening event. According to the IFAR report, the risk of developing BMF and hematological malignancies by 40 years is 90% and 33%, respectively
5). Hematopoietic SCT remains the only treatment to correct the hematologic manifestations in FA patients. Because of high sensitivity to radiation and conditioning agents (alkylating agent), alternative conditioning regimens using lower dose Cy, using Flu and avoiding irradiation have shown hopeful results so far
27).
Recently, a promising outcome using Flu (120-180 mg/m
2), low-dose Cy (40 mg/kg) and ATG based regimens was reported in 8 FA patients. Donors were either related (n=4), or unrelated (n=4). All patients achieved hematopoietic recovery and were alive at median follow up of 72 months (range, 4 to 117 months)
28). In the United Kingdom, a similar nonradiation conditioning regimen consisting of Flu 125-150 mg/m
2, Cy 20-30 mg/kg and ATG was used for 7 FA patients. They were transplanted from unrelated umbilical cord blood (UCB) (n=4), HLA matched unrelated (n=2) and haploidentical (n=1) peripheral blood grafts. All patients were alive at median follow-up of 37 months (range, 13 to 54 months), although 2 rejected their grafts initially, but were rescued by a second transplant
7). In the present study, however, the survival was not different according to the conditioning method possibly secondary to the small number of cases.
SCT from HLA-matched sibling donor generally gives the best outcome, if performed early prior to the development of MDS or leukemia
27). The data from the International Bone Marrow Transplant Registry showed that, among 209 patients transplanted from matched siblings between 1994 and 1999, the 3-year survival was 81% in patients <10 years of age (n=109) and 69% in older patients (n=100)
29).
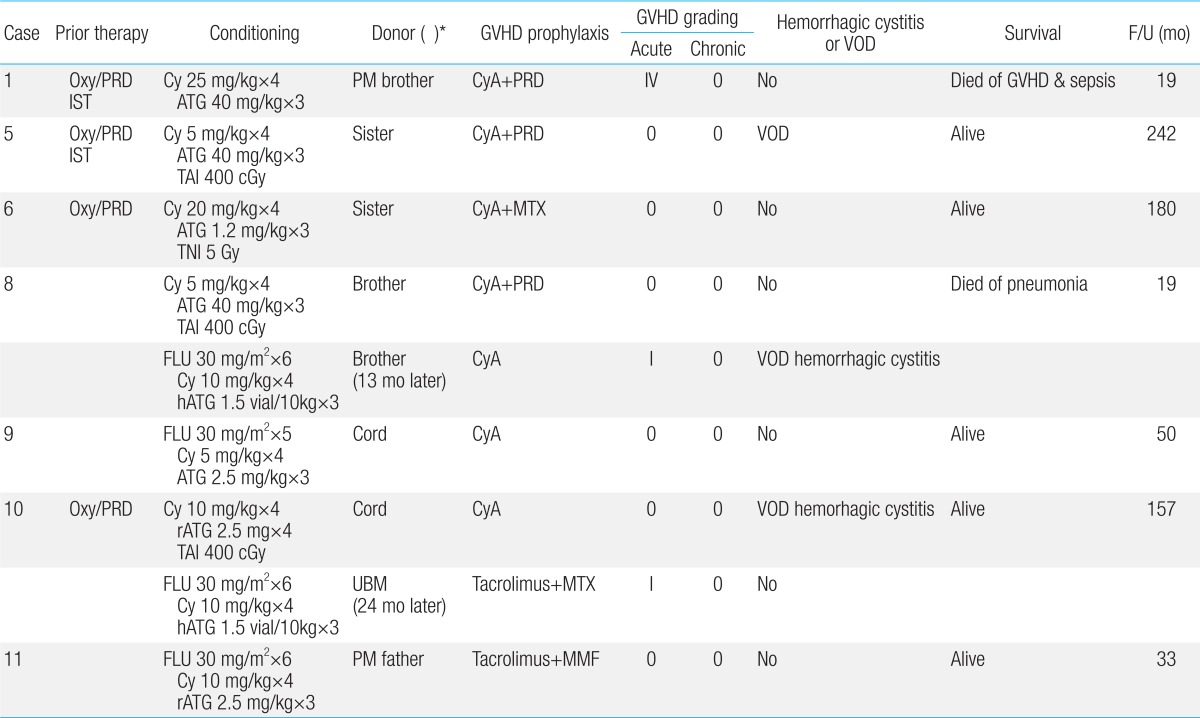
However, the majority of FA patients do not have a HLA matched sibling donor. Until recently, SCT from an alternative donor (i.e., unrelated or HLA mismatched related) has been markedly less successful due to the high rate of graft failure, regimen related toxicity, GVHD and opportunistic infections
27). In the present study, among 2 transplanted from partially matched related donors case 1 died of grade IV acute GVHD and sepsis.
Since the first successful transplant in 1989 for a FA patient
30), UCB has been increasingly used as a donor source. Although the engraftment after UCB transplant (UCBT) was slower, engraftment and survival have been comparable to bone marrow transplant and the incidence of GVHD was reduced
4). In the present study, 2 patients received unrelated UCBT, but both of them rejected initial grafts. Thus, caution should be taken to perform UCBT, especially if the cell number of the UCB unit is marginal or if the patient had received lots of transfusions (case 9).
There are no nation-wide data on FA incidence in Korea, but a recent retrospective study from 9 centers of Korea reported preliminary data on 41 FA patients who underwent SCT between 1996 and 2009
31). Thirty patients (73.2%) were alive with a median of 35 months after transplantation. Flu-based regimens were used in 28 patients (68.3%), and radiation in 21 patients (51.2%). Event-free survival differed by donor types with the best outcome of HLA-matched unrelated donor (79%, n=20), followed by matched sibling donor (67%, n=7), haploidentical parental donor (50%, n=2), and UCB (25%, n=12). The incidence of acute grades II-IV and chronic GVHD was 39% (n=16) and 19% (n=7), respectively.
Many patients who develop BMF initially respond to supportive care such as blood transfusions, androgens, and cytokines (e.g., G-CSF and GM-CSF)
4). Synthetic androgens, such as oxymetholon and danazol, are often considered to be effective for treating BMF in FA patients, although long-term androgen use has side effects that include masculinization, acne, hyperactivity and increased liver tumor incidence
32). In this study, a female patient (case 3) treated with androgen suffered from masculinization and one (case 10) developed peliosis hepatis. Three out of 8 patients treated with androgen showed partial responses. However, all 4 patients who received androgen therapy alone because of the lack of a suitable donor or before the availability of SCT at the institution eventually died. Among 4 patients who finally underwent a SCT, 3 are alive with a median follow up of 173.5 months.
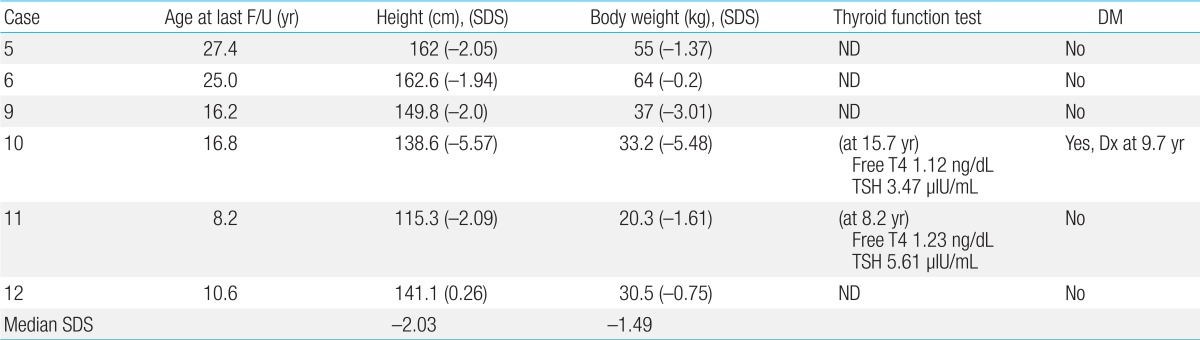
Growth and endocrine abnormalities are clinically important aspects of FA as well. They are often either secondary to hormonal deficiencies, including pituitary hypofunction with hypogonadism, growth hormone deficiency, hypothyroidism and abnormal glucose or insulin metabolism, or treatment-related, such as hemochromatosis with repeated transfusions, or transplant-related complications
3). A thorough baseline and annual endocrine evaluation should be performed in every FA patient to reduce morbidity and improve quality of life.
Despite the small sample sizes and without complementation grouping, the current study provides information on clinical manifestation and long-term outcome of Korean FA patients from a single institution. A nation-wide screening and registry for FA along with genetic subtyping should be initiated (1) to understand the possible ethnic differences in the frequency of subtypes, (2) to unravel genotype-phenotype correlation, (3) to further refine techniques of HSCT, (4) to develop potential future therapies, such as improved gene therapy, or antioxidant compounds, and (5) to establish guidelines for long-term follow-up.




 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


