|
· The number of coronavirus disease 2019 cases has exponentially increased worldwide, and children ≤19 years old account for 11.0% of all confirmed cases.
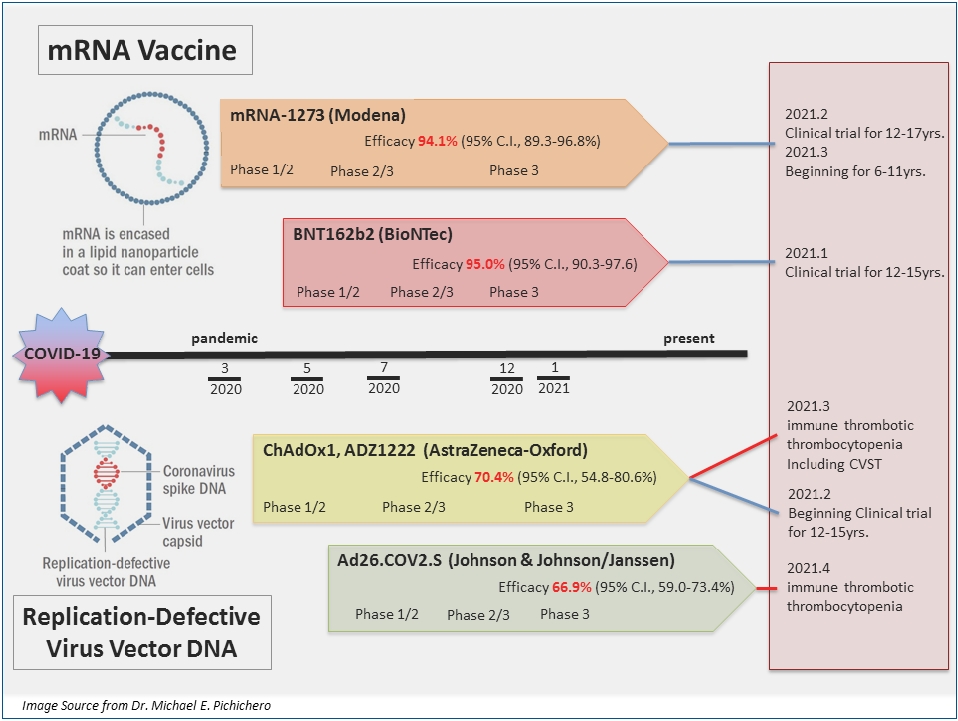
· mRNA vaccines, BNT162b2 and mRNA-1273, and adenoviral vector vaccines, AZD1222 and Ad26.COV2.S, authorized for emergency use in the Emergency Use Listing of the World Health Organization are reviewed.
· Clinical trials of these vaccines have shown that they are safe and serious adverse reactions are rarely observed. |






