Article Contents
| Clin Exp Pediatr > Volume 53(12); 2010 |
Abstract
Purpose
This study aimed to determine the optimal initial vancomycin dose to achieve appropriate trough levels in pediatric patients.
Methods
We analyzed clinical data for 309 children treated with intravenous vancomycin between 2004 and 2009 at 2 different hospitals in South Korea. The patients were 1-16 years old and exhibited normal renal function. Patient data, including reason for treatment and initial dosing regimen, were reviewed. Two subgroups were identified and compared according to initial vancomycin dose: 40 (35-45) mg/kg/day and 60 (55-65) mg/kg/day. Trough levels were obtained at steady state after at least 4 doses of vancomycin.
Results
Patients who received vancomycin had post-operation or wound-related infections (37.2%), localized infection (12.9%), catheter-related infections (9.4%), meningitis (8.7%), or endocarditis (6.8%). Pathogens were confirmed in 79 cases: 28 cases of methicillin-resistant Staphylococcus epidermidis (35.4%) and 25 of methicillin-resistant Staphylococcus aureus (31.6%). Out of the 309 patients, 201 (65%) received vancomycin at 40 mg/kg/day and 108 (35%) at 60 mg/kg/day. Average trough concentrations were significantly different between the groups (P<0.001). Trough levels over 10 mg/L were less likely to be achieved in the 40 mg/kg/day group (14%) than in the 60 mg/kg/day group (49%) (P<0.001). There were no differences in renal function deterioration between the groups.
The clinical use of vancomycin in both adults and children has increased with the emergence of methicillin-resistant Staphylococcus aureus (MRSA)1, 2). A recent report regarding increase of vancomycin minimal inhibitory concentration (MIC) documented treatment failures associated with heterogeneous-vancomycin intermediate S. aureus (hVISA)3, 4). Despite limitations including poor tissue penetration (particularly in the lung), relatively slow bacterial killing, and the potential for toxicity5), vancomycin is regarded as the gold-standard for antibiotic treatment of MRSA infections due to its low cost and established clinical response6).
Major pediatric dosing references for vancomycin recommend a daily dose of 40 mg/kg/day for empiric therapy, while a dose of 60 mg/kg/day is recommended for central nervous system infections7, 8). In adults, the recommended daily dose is 1 g every 12 hours and the recommended trough level is 5 to 10 mg/L9, 10) or 5 to 15 mg/L11).
However, a review recently published by the American Society of Health System Pharmacists (ASHP), the Infectious Diseases Society of America (IDSA), and the Society of Infectious Disease Pharmacists (SIDP) recommended a daily vancomycin dosage of 15 to 20 mg/kg every 8 to 12 hours (based on actual body weight, including obese patients) and maintaining a vancomycin trough concentration between 15 and 20 mg/L for treatment of bacteremia, endocarditis, osteomyelitis, meningitis, and pneumonia12). The purpose of the present study is to evaluate the adequacy of the recommended initial dosage of vancomycin in pediatric patients by analyzing serum trough levels after the initial dosage.
We collected medical records data for children who were treated with intravenous vancomycin therapy for at least five days between January 2004 and September 2009 at Seoul National University Hospital and Seoul National University Bundang Hospital, Seoul, Korea, for this retrospective observational study.
(1) age between 12 months and 16 years; (2) duration of vancomycin infusion ≥5 days; (3) normal renal function; (4) trough level of initial steady state (after at least 4 dose infusion and first data of multiple therapeutic drug monitoring (TDM) data in admission period), and (5) vancomycin dosage range between 35 and 65 mg/kg/day.
(1) abnormal renal function and (2) dosage that was either too low (<35 mg/kg/day) or too high (>65 mg/kg/day). Abnormal renal function patients were defined as patients previously diagnosed with renal disease such as acute renal failure (ARF), chronic renal failure (CRF), or end stage renal disease, or who exhibited abnormal serum creatinine levels for age on the day of initial vancomycin infusion.
Information regarding vancomycin dosage and administration, duration of infusion, trough level, time that the trough level was sampled, diagnosis, culture results, and concomitant other drug therapy was collected by a review of medical records. Demographic data were also collected, including age, sex, weight, and data of serum creatinine, and any other underlying diseases were collected.
In univariate analyses, categorical variables were compared using the chi-square test and continuous variables were compared using the t-test between the two dosage groups. Independent t-tests were also used to compare the mean trough levels between the 40 mg/kg/day and 60 mg/kg/day regimens. Pearson's correlation tests were used to detect correlations between vancomycin trough levels and dosages. P-values <0.05 were considered statistically significant. All analyses were performed using SPSS version 17.0.
The medical records of 502 patients were reviewed, and data for 309 patients were analyzed after exclusions. The mean patient age was 7.62 years and the mean duration of vancomycin infusion was 21.5 days. The patients were divided into two groups. Group A included 201 patients (65%) who received 40 (35-45) mg/kg/day of vancomycin. Group B included 108 patients (35%) who received 60 (55-65) mg/kg/day of vancomycin.
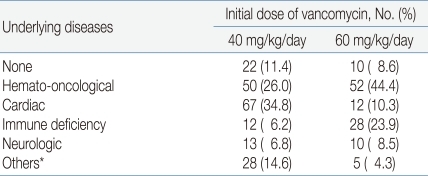
During the study period, vancomycin was recommended to use initial dose of 60 mg/kg/day for the treatment of central nervous system infections, serious infections in cancer patients, or patients who need vancomycin in critical conditions7, 8). Otherwise, vancomycin was used in initial dose of 40mg/kg/day to treat infectious diseases in patients with normal renal function. A total of 277 patients (89.6%) had underlying diseases such as hemato-oncological diseases, cardiac diseases, and immune deficiencies (Table 1).
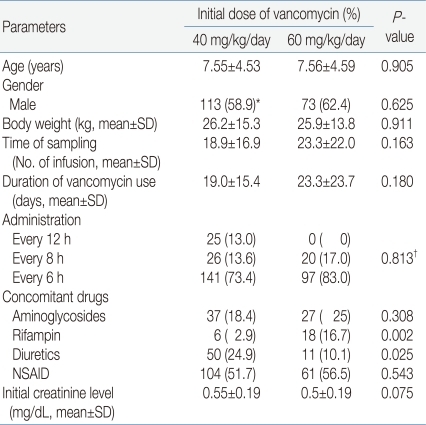
Demographic data of the patients are presented in Table 2. There was no significant difference between the patient groups except concomitant use of rifampicin and diuretics.
Gender, age, body weight, time of trough level sampling, duration of infusion, administration interval (every 6 hours or every 8 hours), initial creatinine level, concomitant use of aminoglycosides and use of non-steroidal anti-inflammatory drugs (NSAIDs) were not significantly different between group A and group B. Concomitant use of rifampin was more frequent in group B (16.7%) than in group A (2.9%) (P=0.002), while concomitant use of diuretics was more frequent in group A (24.9%) than in group B (10.1%) (P=0.025).
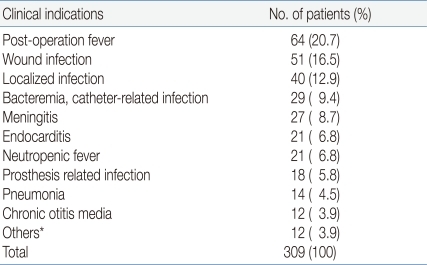
In all patients, vancomycin was administered to treat postoperative fever (20.7%), wound infection (16.5%), localized infections such as abscesses or skin infections (12.9%) and bacteremia (9.4%) or meningitis (8.7%). The indications for which all patients were administered vancomycin are presented in Table 3.
The infectious pathogen was identified in 79 cases. Of these, 28 cases were diagnosed with methicillin-resistant Staphylococcus epidermidis (MRSE) (35.4%) and 25 cases with MRSA (31.6%).
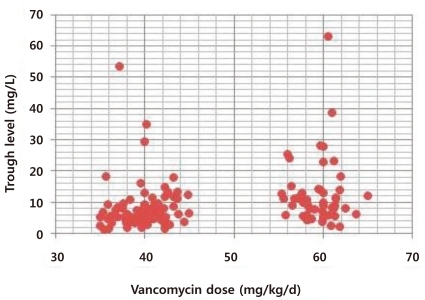
Vancomycin trough levels and initial vancomycin doses were correlated in the total patient sample as shown in Fig. 1 (P=0.000). The mean vancomycin trough level was 6.58±3.52 mg/L (range, 1.3 to 18.4 mg/L) in group A and 12.17±6.34 mg/L (range, 2.3 to 63.1 mg/L) in group B. The mean trough level was significantly lower in group A than in group B (P=0.000).
The distribution of trough level differed significantly according to initial vancomycin dose (Table 4). In group A, 77 patients (38.3%) had trough levels <5 mg/L, 96 patients (47.7%) had trough levels 5-10 mg/L, 20 patients (10%) had trough levels 10-15 mg/L, and only eight patients (4%) had trough levels >15 mg/L. In group B, 22 patients (20.3%) had trough levels >15 mg/L, 31 patients (28.7%) had trough levels 10-15 mg/L, 42 patients (39%) had trough levels 5-10 mg/L and only 13 patients (12%) had trough levels <5 mg/L. In group A, 28 patients (14%) achieved trough levels over 10 mg/L, while in group B, 53 patients (49%) achieved trough levels over 10 mg/L, difference that was statistically significant (P<0.001).
There were no differences in deterioration of renal function between the groups (P=0.609). The creatinine levels of four patients in group A (0.02%) and three patients in group B (0.028%) were higher than normal according to age after vancomycin infusion, but all of these cases normalized after administration of vancomycin was ceased. All seven patients were concomitantly treated with other nephrotoxic drugs, including aminoglycosides and diuretics.
In this study, we investigated the relationships between vancomycin trough concentrations and initial vancomycin dose in 309 pediatric patients with normal renal function. Patients who received dosages of 40 mg/kg/day exhibited mean trough levels that were lower (6.58±3.52 mg/L) than those of patients who received 60 mg/kg/day (12.7±6.34 mg/L), and the proportion of patients maintaining trough levels >10 mg/L was also lower among patients receiving 40 mg/kg/day (14% of group A vs. 49% of group B).
Vancomycin has been used as the primary therapeutic agent to treat MRSA infections for the past 40 years in both adults and children13). Although several recent reports have highlighted the limitations of vancomycin, which include relatively slow bactericidal activity, the development of resistance and associated therapeutic failure, poor tissue penetration, and the potential for serious toxicity5, 14), and newer anti MRSA medications show great promise, vancomycin is well-tolerated and is still the drug of choice to empirically treat all types of MRSA infection1, 15). Until recently, vancomycin was usually administered intravenously to adults at a dosage of 1 g every 12 hours, with the targeted serum trough level set between 5 and 10 mg/L9, 10, 16, 17).
Although the American Thoracic Society advises that vancomycin should be given in doses sufficient to achieve a trough level between 15 to 20 mg/L for the treatment of MRSA pneumonia because of the emergence of MRSA strains with reduced susceptibility to vancomycin, the optimal doses and trough levels in adults remain controversial due to pharmacokinetic and pharmacodynamic properties of vancomycin18), lack of well designed randomized clinical evaluations, and lack of data to support a clear relationship between specific serum concentrations and patient outcome. However, a recent consensus review on therapeutic monitoring of vancomycin recommended maintaining vancomycin trough concentration between 15 and 20 mg/L for bacteremia, endocarditis, osteomyelitis, meningitis, and pneumonia12).
The area-under-the-concentration-time-curve (AUC) divided by the MIC (AUC/MIC) best predicts treatment outcomes when treating invasive MRSA infections with vancomycin in adults, and an AUC/MIC values >400 for vancomycin are associated with optimal outcomes in the treatment of adults19, 20). For pathogens with MICs of 1 mg/L, the minimum trough serum vancomycin concentration would have to be at least 15 mg/L to obtain this target AUC/MIC12). Vancomycin dosages of 15-20 mg/kg given every 8-12 hours are required for most adult patients with normal renal function to achieve the suggested serum concentrations when the MIC is ≤1 mg/L12).
Some data suggest that low serum levels of vancomycin early in the treatment course of MRSA infections may also be associated with the emergence of VISA and hVISA20, 21). Howden et al.22) suggested that not only previous MRSA infection and glycopeptide exposure, but trough serum vancomycin concentrations of <10 mg/L may predict therapeutic failure and the potential for the emergence of VISA or vancomycin-resistant Staphylococcus aureus (VRSA). For this reason, it is recommended that trough serum vancomycin concentrations always be maintained above 10 mg/L to avoid development of resistance by consensus review12).
In pediatric patients, until recently the recommended daily dose of vancomycin was 40 mg/kg/day for common infections, while a dose of 60 mg/kg/day was recommended for serious infections such as central nervous system infections. The recommended trough level was 5-15 mg/L, as in adult patients7, 8).
In a previous study23) of infants and children treated for suspected staphylococcal infections, vancomycin was administered in total dose of 40 mg/kg/day divided into four doses of 10 mg/kg/day every 6 hours. This regimen achieved goal trough levels (5-15 mg/L) in only 45% of 31 control patients. In 33 patients with malignancies, 88% required doses of 60 mg/kg/day to achieve goal trough levels. In another study24), 76 pediatric intensive care unit patients with normal renal function required a mean dose of 60 mg/kg/day to achieve a mean trough level of 7.8 mg/L. These data support the contention that a starting vancomycin dose of 40 mg/kg/day may not be optimal to achieve recommended trough levels in children.
Recently, in a study15) in which pediatric patients received vancomycin doses of 40 mg/kg/day, the AUC/MIC target of 400 was achieved only when the MIC of MRSA isolates was 0.5 g/mL. For MRSA isolates with MIC 1.0 g/mL, the AUC/MIC was always <400 at this dose, suggesting that empiric treatment of invasive MRSA infections in children should implement vancomycin doses of 60 mg/kg/day.
We observed that the mean vancomycin trough level in group A was far short of the recommended trough levels (>10 mg/L) for adults that are required to avoid development of resistance. The results of the present study imply that an initial vancomycin dose 40 mg/kg/day, which is the current standard recommended dose for pediatric patients, may not yield optimal therapeutic effects or prevent the development of antibiotic resistance. However, given only this result, it is too early to confirm that the recommended initial dose of vancomycin for pediatric patients should be higher. A prospective study is warranted exploring the influences of the initial dose of vancomycin and trough levels on the therapeutic results when treating MRSA infections.
This study had several limitations. First, this was a retrospective, observational single center study. Second, we did not evaluate AUC or the MIC because these data were not available for all patients. However, increased serum trough levels are associated with greater AUCs and serum trough levels are considered adequate surrogate markers for AUC, although serum trough levels do not directly reflect AUC. Third, most patients treated with vancomycin had underlying diseases (88.3%), and there were relatively few cases for which the pathogen was microbiologically confirmed (79 cases, 25.6%) of the confirmed cases, only 66.7% were cases of MRSA or MRSE. Therefore we were unable to evaluate microbiological and clinical therapeutic effects of initial vancomycin doses and trough levels. Concomitant use of diuretics such as furosemide were more frequently observed in patients receiving usual dosages (40 mg/kg/day) of vancomycin, and furosemide may influence vancomyin trough levels25-27).
On the other hand, some data indicate that there are no benefits of high dose strategies. Jeffres et al.28) retrospectively evaluated 102 patients with health-care-associated MRSA pneumonia and observed no significant differences in mean vancomycin trough levels or AUC between survivors and nonsurvivors. Similarly, Hermsen et al.6) retrospectively evaluated 55 patients with pneumonia, osteomyelitis, and endocarditis and also observed no significant differences in clinical outcomes between patients who achieved high trough levels (≥15 mg/L) and those with low trough levels (<15 mg/L).
Some published studies report significant associations between increased vancomycin trough concentrations and nephrotoxicity6, 29). However, current consensus is that the nephrotoxic risk of vancomycin is minimal in patients without risk factors for nephrotoxicity, and the relationship of between vancomycin trough concentrations and nephrotoxicity remains controversial because patients receiving aggressive dosing are more likely to receive concomitant nephrotoxins and have other risk factors for nephrotoxicity1). In our study, we did not observe significant association between high initial vancomycin dose and nephrotoxicity.
In conclusion, we observed that traditional recommended doses of vancomycin in pediatric patient (40 mg/kg/day) was insufficient to achieve recommended trough levels for therapeutic effect (5-10 mg/L). However, before recommending increases in the initial vancomycin doses for pediatric patients, further studies are necessary to clarify the relationship between vancomycin trough levels and clinical efficacy, as well as the possibilities of nephrotoxicity in children.
References
1. Hazlewood KA, Brouse SD, Pitcher WD, Hall RG. Vancomycin associated nephrotoxicity: grave concern or death by character assassination? Am J Med 2010;123:182.e1–182.e7.



2. Kaplan SL, Hulten KG, Gonzalez BE, Hammerman WA, Lamberth L, Versalovic J, et al. Three-year surveillance of community-acquired Staphylococcus aureus infections in children. Clin Infect Dis 2005;40:1785–1791.


3. Sakoulas G, Moellering RC Jr. Increasing antibiotic resistance among methicillin-resistant Staphylococcus aureus strains. Clin Infect Dis 2008;46:S360–S367.


4. Musta AC, Riederer K, Shemes S, Chase P, Jose J, Johnson LB, et al. Vancomycin MIC plus heteroresistance and outcome of methicillin-resistant Staphylococcus aureus bacteremia: trends over 11 years. J Clin Microbiol 2009;47:1640–1644.



5. Kollef MH. Limitations of vancomycin in the management of resistant staphylococcal infections. Clin Infect Dis 2007;45:S191–S195.


6. Hermsen ED, Hanson M, Sankaranarayanan J, Stoner JA, Florescu MC, Rupp ME. Clinical outcomes and nephrotoxicity associated with vancomycin trough concentrations during treatment of deep-seated infections. Expert Opin Drug Saf 2010;9:9–14.


7. Taketomo CK, Hodding JH, Kraus DM. Pediatric Dosage Handbook. 2007;14th ed. Hudson, OH: Lexi-Comp, :1596–1598.
8. Schaad UB, McCracken GH Jr, Nelson JD. Clinical pharmacology and efficacy of vancomycin in pediatric patients. J Pediatr 1980;96:119–126.


9. Patanwala AE, Norris CJ, Nix DE, Kopp BJ, Erstad BL. Vancomycin dosing for pneumonia in critically ill trauma patients. J Trauma 2009;67:802–804.


11. Ambrose P, Winter ME. Vancomycin. Basic Clinical Pharmacokinetics. 2004;4th ed. Philadelphia: Lippincott Williams and Wilkins, :451–476.
12. Rybak M, Lomaestro B, Rotschafer JC, Moellering R Jr, Craig W, Billeter M, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 2009;66:82–98.


13. Pillai SK, Wennersten C, Venkataraman L, Eliopoulos GM, Moellering RC, Karchmer AW. Development of reduced vancomycin susceptibility in methicillin-susceptible Staphylococcus aureus. Clin Infect Dis 2009;49:1169–1174.


15. Frymoyer A, Hersh AL, Benet LZ, Guglielmo BJ. Current recommended dosing of vancomycin for children with invasive methicillin-resistant Staphylococcus aureus infections is inadequate. Pediatr Infect Dis J 2009;28:398–402.



18. American Thoracic Society. Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388–416.


19. Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infection. Clin Pharmacokinet 2004;43:925–942.


20. Charles PG, Ward PB, Johnson PD, Howden BP, Grayson ML. Clinical features associated with bacteremia due to heterogeneous vancomycin-intermediate Staphylococcus aureus. Clin Infect Dis 2004;38:448–451.


21. Sakoulas G, Gold HS, Cohen RA, Venkataraman L, Moellering RC, Eliopoulos GM. Effects of prolonged vancomycin administration on methicillin-resistant Staphylococcus aureus (MRSA) in a patient with recurrent bacteraemia. J Antimicrob Chemother 2006;57:699–704.


22. Howden BP, Ward PB, Charles PG, Korman TM, Fuller A, du Cros P, et al. Treatment outcome for serious infections caused by methicillin-resistant Staphylococcus aureus with reduced vancomycin susceptibility. Clin Infect Dis 2004;38:521–528.


23. Chang D. Influence of malignancy on the pharmacokinetics of vancomycin in infants and children. Pediatr Infect Dis J 1995;14:667–673.


24. Glover ML, Cole E, Wolfsdorf J. Vancomycin dosage requirements among pediatric intensive care unit patients with normal renal function. J Crit Care 2000;15:1–4.


25. Pea F, Porreca L, Baraldo M, Furlanut M. High vancomycin dosage regimens required by intensive care unit patients cotreated with drugs to improve haemodynamics following cardiac surgical procedures. J Antimicrob Chemother 2000;45:329–335.


26. Nivoche Y, Contrepois A, Cremieux AC, Carbon C. Vancomycin in rabbits: pharmacokinetics, extravascular diffusion, renal excretion and interactions with furosemide. J Pharmacol Exp Ther 1982;222:237–240.

27. Pea F, Furlanut M. Pharmacokinetic aspects of treating infections in the intensive care unit. Clin Pharmacokinet 2001;40:833–868.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


