Article Contents
| Korean J Pediatr > Volume 56(3); 2013 |
Abstract
Purpose
Anthracyclines have been utilized in the treatment of children with acute lymphoblastic leukemia (ALL). Recent studies have shown that anthracyclines may induce toxicity in the vascular endothelium. This study was performed using brachial artery reactivity (BAR) to evaluate vascular endothelial function in ALL patients who were treated with anthracycline chemotherapy.
Methods
We included 21 children with ALL who received anthracycline chemotherapy and 20 healthy children. The cumulative dose of anthracyclines in the ALL patients was 142.5±18.2/m2. The last anthracycline dose was administered to the patients 2 to 85 months prior to their examination using BAR. The diameter of the brachial artery was measured in both groups using echocardiography, and BAR was calculated as the percentage change in the arterial diameter after release of the cuff relative to the baseline vessel diameter.
Results
In the anthracycline-treated group, BAR was observed to be 3.4%±3.9%, which was significantly lower than that observed in the control group (12.1%±8.0%, P<0.05). The time elapsed after the last anthracycline treatment and the age at the time of treatment did not affect the change in BAR (P=0.06 and P=0.13, respectively).
Conclusion
These results provided evidence that treatment of ALL patients with anthracycline results in endothelial dysfunction. A larger cohort study and a longer follow-up period will be required to clarify the relationship between endothelial dysfunction resulting from anthracycline treatment for childhood ALL and occurrence of cardiovascular diseases later in life.
The development of new supportive agents and antibiotics has facilitated the use of more intensified and effective anticancer drugs which enhance the long term survival of childhood acute lymphoblastic leukemia (ALL) patients1). With the increased survival rate of children with ALL, attention has now shifted toward the long-term complications of this intensified treatment2).
Lately the scientific interest in evaluating the impact of vascular endothelial dysfunction in ALL is increasing. ALL itself, chemotherapeutic agents, or other conditions (e.g., sepsis) can result in the vascular endothelial dysfunction3). Among chemotherapeutic agents anthracyclines in particular have been shown to have potential to cause vascular endothelial dysfunction4,5).
The vascular endothelium is an active, dynamic tissue which controls many important functions including secretion of factors regulating vascular tone, platelet aggregation and leukocyte interactions. Endothelial dysfunction is an important factor in the development of atherosclerosis, hypertension, coronary artery disease and heart failure6). In this regard, anthracyclines could be a causative agent related to the pathogenesis of atherosclerosis or other cardiovascular diseases.
Vascular endothelial function can be characterized by flow-mediated vasodilatation of the brachial artery, which is measured by comparing the brachial artery diameter at rest to the diameter after increased forearm blood flow. Over the past decade, noninvasive technique, brachial artery reactivity (BAR), has been developed in order to evaluate vascular endothelial function7). Although there are some technical and interpretive limitations, BAR has been widely used to evaluate the vascular endothelial function in patients with cardiovascular diseases including artherosclerosis, diabetes mellitus, hypercholesterolemia, coronary artery disease and peripheral vascular disease8.9).
In this study, we used BAR in order to evaluate the function of the vascular endothelium in patients to whom had been administered chemotherapy with anthracyclines for the treatment of childhood ALL.
An informed consent was obtained from the guardians of all enrolled participants and the study protocol was approved by the Gachon Medical School Ethics Committee in October, 2010. Twenty-one ethnic Korean children, including 11 boys and 10 girls aged 4 years to 18 years with a median age of 11 years, who had been newly diagnosed with high risk ALL were enrolled onto this study and administered anthracyclines at the Pediatrics Department of Gil Hospital, Gachon University in Incheon, Korea. The enrolled patients were treated with a dosing regimen consisting of induction, consolidation with cranial irradiation, interim maintenance, a single delayed intensification and a maintenance phase. Daunomycin was administered in the induction phase and doxorubicin was administered in the delayed intensification phase. As a consequence, the cumulative dose of anthracyclines was 142.5±18.2/m2 prior to the maintenance phase. The other chemotherapeutic agents given to the patients included vincristine, prednisolone, L-asparaginase, cyclophosphamide, 6-mercaptopurine, methotrexate, intrathecal cytosine arabinoside and methotrexate. We excluded the participation of two patients who had existing conditions known to influence BAR results including hypertension, hyperlipidemia, diabetes mellitus, smoke exposure, cardiovascular surgery or peripheral vascular disease. We performed the BAR test with enrolled subjects who had completed their scheduled therapy or were in the maintenance phase after the delayed intensification treatment. A control group of 20 ethnic Korean children composed of 9 boys and 11 girls, aged 2 years to 16 years with a median age of 9 years, were enrolled in this study. In addition, we selected a control group who were healthy and without any major medical illness or conditions known to influence BAR results.
The demographic characteristics of both the control and treatment groups are shown in Table 1. The differences in age and gender between the groups were found to be negligible. In addition, physical characteristic differences including height and body weight between the groups were also found to be negligible.
All investigations were carried out using a 5/12-MHz linear array ultrasound device (ATL 3000, Philips Healthcare, Bothell, WA, USA). A single experienced pediatric cardiologist (DYC), blinded to the subject's status, measured the vessel diameter. Each subject remained in a supine position on the examination table for at least 5 minutes prior to testing. The baseline blood pressure was obtained using a manual blood pressure cuff. Electrocardiographic leads were connected to the patient and the signal was displayed on a monitor. The baseline images of the brachial artery were obtained approximately 5 cm proximal to the antecubital crease. Hyperemia was induced by inflating the blood pressure cuff on the arm in order to occlude arterial flow (40 mmHg above the measured resting systolic blood pressure) for 5 minutes, and then rapidly deflating the cuff. The reactive hyperemia images were obtained 1 minute after cuff deflation. The diameter of the brachial artery was measured from anterior to posterior and between the media and adventitia (m-line) at the end diastolic. The arterial mean diameter was averaged from the images obtained during the three cardiac cycles synchronized with the R-wave peaks on the electrocardiogram. BAR was calculated as the percentage change of arterial diameter after release of the cuff relative to the baseline vessel diameter.
Results were expressed as mean±standard deviation. Comparisons between the two groups were performed using an independent samples t-test, Mann-Whitney test and chi-square test. The variables were evaluated by Spearman's rank correlation. For all analyses, P<0.05 was accepted as statistically significant. All statistical analyses were performed using MedCalc ver. 12.3.00 (MedCalc, Mariakerke, Belgium).
Occlusive pressure was 150.4±14.1 mmHg in the anthracyclines treated group and 148.9±10.7 mmHg in the control group (P=1.0). Resting vessel diameter was 3.3±0.6 mm in the anthracyclines treated group and 3.1±0.4 mm in the control group (P=0.36). In the anthracyclines treated group, the average BAR was 3.4%±3.9%. The control group had an average BAR of 12.1%±8.0% (Table 2). The difference in BAR between these groups was statistically significant (P<0.05) (Fig. 1).
There was no correlation between the elapsed time after the last anthracyclines administration and BAR among the treatment group (r=0.42, P=0.06) (Fig. 2).
There was no correlation between the patient age at time of anthracyclines administration and BAR among the treatment group (r=0.34, P=0.13) (Fig. 3).
With advances in treatment, the survival rate of childhood leukemia cases has significantly improved. However, the increase in the childhood leukemia survival rate is accompanied by long term adverse effects impacting the quality of life for adult survivors10). The survival rate of children diagnosed with ALL has been improved with the use of anthracyclines, yet their use is accompanied by high risk factors1). The use of anthracyclines is known to be related to the development of irreversible cardiomyopathy, and this development occurs more frequently in cases of high cumulative doses. In recent years, this potent antineoplastic drug has been reported to produce toxicity affecting the vascular endothelium4,5). Vascular endothelial cells, lining the entire circulatory system, have distinct and unique functions affecting maintenance of blood circulation and fluidity as well as regulation of vascular tone, coagulation, and inflammatory responses. Therefore an alteration of regulatory function of vascular endothelium by damage is related to the development of cardiovascular disease, including atherosclerosis and hypertension11). Damage of the vascular endothelium caused by diverse insults such as antiendothelial antibodies, disturbed cytokines, viral infections, free radical formation or oxidation of lipids, has been implicated in cardiovascular diseases, including atherosclerosis and hypertension11).
Recent research has revealed that anthracyclines induce vascular endothelial damage by a multifactorial process12). The main mechanism leading to damage is the inhibition of endothelial nitric oxide synthase (eNOS) due to the action of anthracyclines binding to the reductase domain of eNOS13). As a consequence, there is not only a reduction in nitric oxide (NO) production but also an increase in superoxide generation. Decreased availability of NO, which is the main vasodilator and the regulator of other protective properties of the endothelium, diminishes vascular reactivity. In addition, the increase in free radicals may play a role in the apoptosis of vascular endothelial cells14,15). These events could be related to the development of cardiovascular diseases including atherosclerosis and hypertension, and lead to heart failure.
Evaluation of BAR, an endothelium-dependent function, has been used to evaluate vascular endothelial function7,8). In practice, a measure of BAR in breast cancer patients undergoing chemotherapy with adriamycin (60 mg/m2) revealed a significant reduction in flow-mediated dilation from 6.5%±1% (before administration) to 2.5%±1% (just after administration)4). In addition, serum nitrite and nitrate concentrations fell after adriamycin administration, indicating insufficient NO synthesis. Another recent study, examining childhood cancer patients treated with a cumulative dose of more than 300 mg/m2 of anthracyclines demonstrated that the BAR for these patients significantly decreased as compared to a healthy control group as 3.8%±3.4% and 6.7%±3.3%, respectively5). Our study of childhood ALL patients treated with anthracyclines also showed similar results whereby BAR was 3.4%±3.9% in the treatment group and 12.1%±8.0% in the control group. In two previous human studies, the anthracyclines doses administered were 60 mg/m2 to over 300 mg/m2, however, both of those studies showed that patient BAR results diminished. The administered dose of anthracyclines in our study was around 140 mg/m2 and also resulted in diminished BAR results. These results suggested that the anthracyclines dose does not affect BAR results, although evaluation of the toxicity of doxorubicin as it effects vascular endothelium using the organ culture method revealed that it induces apoptotic cell death followed by impairment of endothelium-dependent relaxation in a dose- and time-dependent manner12). A larger cohort study is needed in order to evaluate the magnitude of dose which provokes injury to the vascular endothelium.
It is not clear yet that anthracyclines induce vascular endothelial damage by rapid-onset process or mechanism which needs time such as apoptosis. In our study BAR was not related to the elapsed time after the last anthracyclines was administered. The small numbers of patients enrolled in the study might affect the result of the relationship between final treatment time and BAR findings. Further studies in a larger group of patients and with a long term follow up period are required.
It is unknown whether or not age is a significant factor in the vulnerability of vascular endothelium to the toxic effects of anthracyclines. In our study, the age at anthracyclines administered did not affect BAR results, indicating that the age factor was not important in the endothelial dysfunction induced by anthracyclines.
Although we investigated endothelial dysfunction in the brachial artery, which is a superficial artery, vasodilator changes in the brachial artery correlate well with coronary artery function16). Our study results suggested that the endothelial dysfunction due to treatment may be related to and may play an important role in the progression of cardiovascular disease. In addition to endothelial dysfunction, survivors of childhood ALL suffer cardiovascular risk factors such as obesity, hyperlipidemia, physical inactivity, insulin resistance and metabolic syndrome17). From a clinical point of view, children treated with anthracyclines are at risk of early cardiovascular disease. Thus, cardiovascular disease risk factors such as hypertension, hypercholesterolemia, smoking, diabetes and obesity should be monitored and controlled.
In this human study, it was revealed that the around 140 mg/m2 dose of anthracyclines induces the vascular endothelial dysfunction in ALL patients. A limitation of this study is that the other which may effect on vascular endothelium, such as other chemotherapeutic agents used in conjunction with anthracyclines and cranial radiation therapy and metabolic problems by ALL itself, were not considered. As the endothelial independent dilation was not evaluated, we could not determine whether a defect in the smooth muscle contributed to the decrease in BAR. The small number of patients enrolled in this study might affect the result of this study. Also, gender which might affect the BAR was not evaluable because the enrolled subjects were small.
Additional follow-up over many years would be necessary in order to confirm that endothelial dysfunction in anthracyclines treated patients is associated with subsequent cardiovascular diseases.
References
2. Bhatia S. Late effects among survivors of leukemia during childhood and adolescence. Blood Cells Mol Dis 2003;31:84–92.


3. Hatzipantelis ES, Athanassiou-Metaxa M, Gombakis N, Tzimouli V, Taparkou A, Sidi-Fragandrea V, et al. Thrombomodulin and von Willebrand factor: relation to endothelial dysfunction and disease outcome in children with acute lymphoblastic leukemia. Acta Haematol 2011;125:130–135.


4. Duquaine D, Hirsch GA, Chakrabarti A, Han Z, Kehrer C, Brook R, et al. Rapid-onset endothelial dysfunction with adriamycin: evidence for a dysfunctional nitric oxide synthase. Vasc Med 2003;8:101–107.


5. Chow AY, Chin C, Dahl G, Rosenthal DN. Anthracyclines cause endothelial injury in pediatric cancer patients: a pilot study. J Clin Oncol 2006;24:925–928.


7. Corretti M. Brachial artery reactivity: clinical tool or research toy? J Am Soc Echocardiogr 2004;17:693–696.


8. Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 2002;39:257–265.

9. Lieberman EH, Gerhard MD, Uehata A, Selwyn AP, Ganz P, Yeung AC, et al. Flow-induced vasodilation of the human brachial artery is impaired in patients <40 years of age with coronary artery disease. Am J Cardiol 1996;78:1210–1214.


10. Pui CH, Pei D, Sandlund JT, Campana D, Ribeiro RC, Razzouk BI, et al. Risk of adverse events after completion of therapy for childhood acute lymphoblastic leukemia. J Clin Oncol 2005;23:7936–7941.


11. Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 1993;362:801–809.


12. Murata T, Yamawaki H, Yoshimoto R, Hori M, Sato K, Ozaki H, et al. Chronic effect of doxorubicin on vascular endothelium assessed by organ culture study. Life Sci 2001;69:2685–2695.


13. Vásquez-Vivar J, Martasek P, Hogg N, Masters BS, Pritchard KA Jr, Kalyanaraman B. Endothelial nitric oxide synthase-dependent superoxide generation from adriamycin. Biochemistry 1997;36:11293–11297.


14. Kalivendi SV, Kotamraju S, Zhao H, Joseph J, Kalyanaraman B. Doxorubicin-induced apoptosis is associated with increased transcription of endothelial nitric-oxide synthase. Effect of anti-apoptotic antioxidants and calcium. J Biol Chem 2001;276:47266–47276.


15. Wu S, Ko YS, Teng MS, Ko YL, Hsu LA, Hsueh C, et al. Adriamycin-induced cardiomyocyte and endothelial cell apoptosis: in vitro and in vivo studies. J Mol Cell Cardiol 2002;34:1595–1607.


Fig. 1
Brachial artery reactivity was higher in the control group than in the anthracycline-treated group (P<0.05). The vertical bar represents the values from the lower to upper quartile (25th to 75th percentile). The middle line represents the mean. The line extending between the minimum and maximum values includes the outside and far out values. The circles represent each value. BAR, brachial artery reactivity.
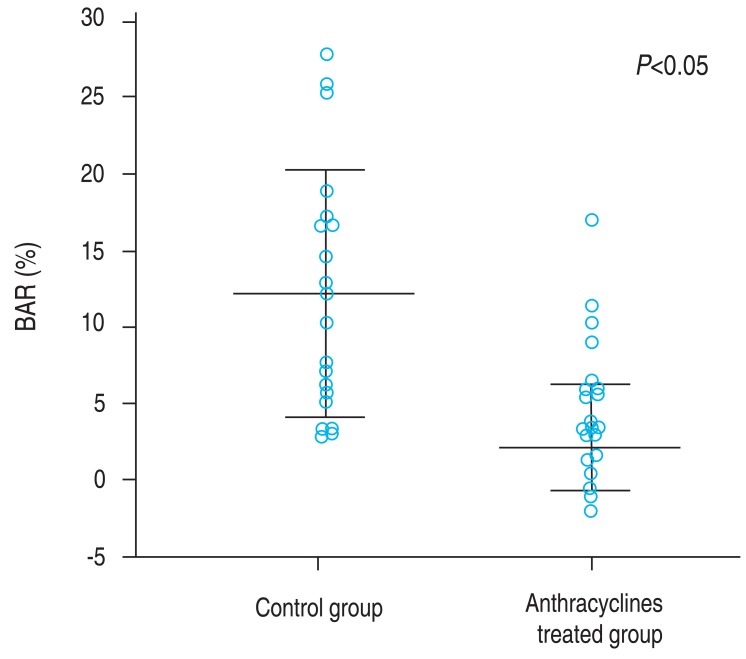
Fig. 2
The correlation between elapsed time after the final anthracycline treatment and brachial artery reactivity (BAR) was not statistically significant (r=0.42, P=0.06).
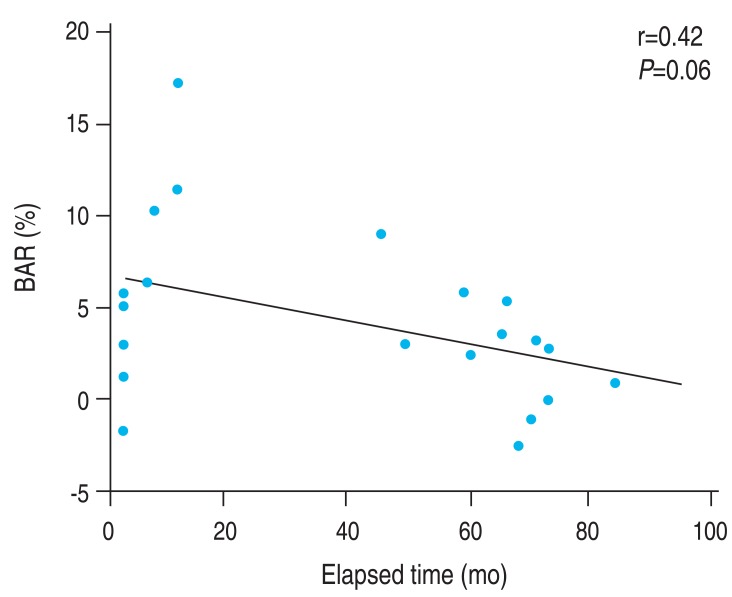
Fig. 3
The correlation between patient age at anthracycline administration and brachial artery reactivity (BAR) was not statistically significant (r=0.34, P=0.13).






 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


