Article Contents
| Korean J Pediatr > Volume 56(4); 2013 |
Abstract
Purpose
Apical muscular ventricular septal defects (MVSDs), especially in small infants, can be difficult to manage using surgical and percutaneous closure. An intraoperative perventricular procedure is a good option for closing apical MVSDs in small children with or without associated cardiac anomalies. We evaluated the results of hybrid perventricular closure of apical MVSDs performed using an Amplatzer duct occluder (ADO).
Methods
We retrospectively reviewed the medical records of 5 patients who underwent hybrid perventricular closure of MVSDs with ADOs, from March 2006 to May 2011. The median patient age at the time of the procedure was 12 months (range, 25 days to 25 months), and the median body weight was 9.1 kg (range, 4.3 to 15 kg). Two patients had multiple ventricular septal defects (VSDs; additional perimembranous VSD in 1 patient and multiple MVSDs in the other) and 3 patients had associated cardiac anomalies; complete transposition of the great arteries in 1 patient and an atrial septal defect in 2 patients. All the procedures were performed on beating hearts, exception in 1 case. The ADO selected for the aortic side was at least 1 to 2 mm larger than the largest VSD in the left ventricle side.
A variety of surgical procedures have been described to close the muscular ventricular septal defects (MVSDs)1). But the incidence of residual defects remains high after surgery, especially if is located in the apical septum2). Lock et al.3) first reported their experience of a transcatheter closure of ventricular septal defect (VSD) using a Rashkind double umbrella in 1987. After the introduction of the Amplatzer MVSD occluder (AMVSDO) in 1998, several authors4-6) reported their initial application of the device for single muscular VSD in children and adults. Since the first report by Amin et al.7) in 1998, a perventricular device closure of VSD has been attempted in a variety of situations such as small infants with large VSD or infants who had associated cardiac anomalies requiring surgical correction. The application of closure devices designed for implantation in an interatrial septal defect or a patent ductus arteriosus (PDA) has been sporadically reported8-10). The Amplatzer duct occluder (ADO; AGA Medical, Golden Valley, MN, USA) has been used to close a ruptured aneurysm of the sinus of valsalva with no untoward effect11). Pedra et al.12) demonstrated the feasibility of implanting an ADO for closure of MVSD. A MVSD closure using ADO was first reported in 200513,14). Nowadays an ADO is also used for closure of perimembranous VSD (PMVSD).
In this article, we report our experience of perventricular closure of apical MVSD using ADO.
Between March 2006 and May 2011, we performed perventricular MVSD closures with ADO in 5 patients (3 females and 2 males) and their medical records were reviewed retrospectively. The data of the patients and their associated cardiac anomalies are described in Table 1. Their median age was 12 months (range, 25 days to 25 months) and median body weight was 9.1 kg (range, 4.3 to 15 kg). All patients had apical MVSD or VSDs which were difficult to approach surgically and the associated cardiac problems requiring surgery except for one. Two patients had multiple VSDs. Three patients had associated cardiac anomalies which were complete transposition of the great arteries (TGA) in one, and atrial septal defect (ASD) in two. Three patients had previous cardiac surgery, including pulmonary artery banding in two, and PDA ligation in two patients. The size of the MVSDs on the left ventricle (LV) side, assessed by intraoperative transesophageal echocardiography (TEE), ranged from 5.0 to 7.0 mm with a median size of 6.4 mm, and the aortic disc size of the ADO ranged from 8 to 10 mm. The median ratio of ADO/defect size was 1.6 (range, 1.4 to 2.5)/1.
The technique of transcatheter closure of MVSDs that was used was similar to that described by Michel-Behnke et al.15) and the procedures are described in Figs. 1, 2. All procedures were done in the operating room under general anesthesia and TEE guidance. An appropriate device size was chosen for the aortic side to be at least 1 to 2 mm larger than the maximum VSD size on the LV side. The defect size was measured by intraoperative TEE in all patients. The largest VSD was selected to close in patients who had multiple VSD. We used a larger sized ADO in patients with multiple MVSD. With the exception of one case, all procedures were performed on beating hearts without cardiopulmonary bypass (CPB). The heart was approached via a median sternotomy in four patients or subxyphoid incision in one patient who had an isolated apical MVSD. The right ventricular (RV) puncture site was chosen by tipping the RV wall with a blunt instrument to know the way from the RV puncture site to MVSD to avoid trauma to major chordae or a papillary muscle. After passing a wire and sheath through the MVSD into the LV cavity, a purse-string suture was placed. We used a short arterial introducer as a sheath instead of an original long sheath (Fig. 2A, B). The arterial introducer was selected as 1 French larger one than the recommended long sheath size for the selected ADO. The device was advanced through the short sheath into the LV and the aortic disc was deployed (Fig. 2C) under TEE guidance. The partially deployed occluder and sheath were pulled back to the septum (Fig. 2D) and configured with the pulmonic disc in the RV adjacent to the septum (Fig. 2E). After confirmation of the device position and residual shunt, the ADO was detached and the sheath was removed (Fig. 2F). The puncture site was closed by a purse string suture. All patients were given an anti-platelet dose aspirin for 6 months following the procedure.
After device closure, surgeons performed additional procedures for the associated cardiac anomalies or problems related with previous procedures under CPB during the same session in four patients.
All procedures were successful with the devices at the desired position. For early follow-up, all of patients had residual leakage upon localization of the occluder. During the follow-up (median 2.4 years) all patients were doing well with no signs of significant shunting, the initially assessed apical septal defect was completely closed in three of five patients and a small residual VSD was noted in two patients who had multiple VSDs. Additional procedures were performed on CPB in four patients who had associated cardiac anomalies during the same session after device closure. This included an arterial switch operation, patch closure of additional VSD, ASD closure, tricuspid valve plasty and debanding of the pulmonary artery.
There was no patient who showed LV systolic dysfunction immediately and late after the procedure. We did not much pay attention to diastolic function immediately after the procedure, but no patient showed evidence of LV diastolic dysfunction at the last echocardiography. No patient showed gross RV dysfunction after the procedure although we did not measure specific parameters of RV function.
One patient who had mild mitral regurgitation (MR) with mild prolapse showed gradual progression to moderate degree of MR during 6 years of follow-up. No other patient had significant amount of MR after the procedure.
Two patients showed increase of QRS duration on postprocedure electrocardiograms (from about 60 to 90 ms in one, from about 80 to 100 ms in the other). No other electrocardiographic change was found except regression of ventricular hypertrophy in our patients. No patient showed change of device position on follow-up chest X-rays or echocardiograms.
In patient 1, the cardiology doctors were called to the operation room after surgeons had already started the CPB. Therefore, the MVSD closure was done on a nonbeating heart and we couldn't pass a guide wire through the VSD by perventricular approach. Fortunately we successfully found access to the LV from the right atrium (RA) although the MVSD could not be seen clearly from the RA. A short sheath was introduced across the tricuspid valve (TV) into the RV and finally to the LV over the guide wire.
An intraprocedural complication occurred in the patient 4 (aged 25 days and body weight was 4.3 kg). This patient had a complete TGA and multiple apical MVSD. In that patient, the short sheath went too deep in his small LV cavity, which might have aroused entanglement of the ADO aortic disc with mitral valve chordae or the LV lateral wall damage. Although we somewhat felt resistance while pulling the whole system back (Fig. 3A), we could attach the aortic disc of the ADO to the interventricular septum. However the sheath slipped out of the heart while we pulled the sheath back to open the pulmonic side of the ADO. We thought it was at least partly because the RV puncture site was too close to the interventricular septum and we therefore retried at another site of the RV wall. Immediately after the successful closure of the muscular VSD, we found LV lateral wall tearing and bleeding which required surgical sutures of the LV wall. After that, he underwent an arterial switch operation without further problems and had postoperative course that was uneventful. An echocardiography before discharge showed pseudoaneurysm or fluid cavity at the apical area (Fig. 4A), but LV function was good without regional wall motion abnormality. The pseudoaneurysm regressed gradually (Fig. 4B) and disappeared at 20 months after the procedure. This patient had no significant MR after the procedure. There was no other complication during the procedure and follow-up. At a median follow-up of 2.4 years (range, 0.5 to 5 years), all patients were doing well with no signs of significant shunting (no leakage in 3 patients, minimal leakage in 2 patients).
How to treat a sizable apical MVSD can be a difficult decision, especially for small patients with associated cardiac problems. The surgical repair of multiple MVSDs is associated with considerable mortality and morbidity1,2). The surgical approach from the right side of the heart doesn't allow for adequate visualization, and this is related with significant residual of the VSD shunt16). The surgical approach from the LV side provides an adequate field of vision, but it requires a left ventriculotomy which is related with long-term left chamber dysfunction, arrhythmias and apical aneurysm17-19). Transcatheter closure of an apical MVSD is a valuable alternative to the operation. It has been proven to be effective20-22). However, the transcatheter closure may require the use of large venous sheaths for device delivery, and exposes small infants to the risk of peripheral vascular injury and intervening cardiac structure damage. An intraoperative perventricular procedure can be a good option for closure of apical MVSDs in small children with or without associated cardiac anomalies. A perventricular closure of MVSD was first attempted in a canine model in 1998, followed by the successful use of this device in an 8-month-old-baby by Amin et al.7) in the same year. A perventricular device closure of apical MVSD has been described with good results23-25). Since Amin's first report in 1998, the AMVSDO was introduced for muscular defects not only in congenital heart defects but also effectively in postmyocardial infarction VSD26-28). In relation with the particular VSD shape, an ADO has been the preferred choice by some authors29-31). MVSD is usually smaller in the RV side as compared to the LV, and often multiple in the RV side even in case with single in the LV side. We thought, therefore, that ADO which has a trapezoidal profile and a smaller pulmonic end without a RV side retention disc would be good for apical MVSD closure, especially in small infants. Also this device costs less than AMVSDO. On the other hand, the characteristics of deficient RV side retention disc of the ADO may lead to device instability or mobilization after closure of muscular VSD. We, however, presumed that device instability would not be the problem because LV pressure is higher than the RV pressure and muscular VSD size has tendency to be smaller in RV side.
In our experience, we met two technical complications for the hybrid closure of an apical MVSD. One thing is how to prevent the LV wall or mitral valve apparatus injury by the delivery system or the device. In neonates or small infants, the LV dimension is so small that safe space for manipulation during the procedure is very limited. It is essential to monitor the position of the delivery system and the device, and its relation with the LV wall and mitral valve apparatus throughout the procedure. Actually, one complication occurred for one patient who had complete TGA and multiple MVSD as well as apical MVSD. We think the LV lateral wall damage in that patient was associated with too deep an aortic disc position in the LV. In that patient, the damage could easily be repaired making this one of the benefits of the hybrid approach. Another technical issue is choosing the best location of the RV puncture site. If the puncture site is too close to the apical MVSD, selection of VSD may be easy. However it is more difficult to keep the sheath in adequate position while pulling back the whole system after deployment of the aortic disc in the LV because the sheath can easily be slipped out of the RV. Therefore we think it is better to keep some distance from the RV puncture site to a targeted MVSD.
Although we have attempted this hybrid closure of apical MVSD primarily in patients with associated cardiac problems requiring the surgical procedure, an isolate of apical MVSD patients can have benefits from this approach such as short procedure time, avoidance of CPB, small surgical scar, and immediate handling for possible complications. The limitations of this study include a small patient sample, short follow-up, and the lack of randomization.
In conclusion, in our small series, the hybrid approach with the ADO was an effective option for apical MVSD closure. Although we should pay more attention to the delivery system and device position and select the RV puncture site more carefully in small infants, it is expected that we can do this procedure with low complication rate if only we keep several important points under consideration. With more experience, perventricular closure using an ADO can provide effective, one-stage repair in small infants with large apical MVSD and associated cardiac problems.
References
1. Kitagawa T, Durham LA 3rd, Mosca RS, Bove EL. Techniques and results in the management of multiple ventricular septal defects. J Thorac Cardiovasc Surg 1998;115:848–856.


2. Serraf A, Lacour-Gayet F, Bruniaux J, Ouaknine R, Losay J, Petit J, et al. Surgical management of isolated multiple ventricular septal defects. Logical approach in 130 cases. J Thorac Cardiovasc Surg 1992;103:437–442.


3. Lock JE, Block PC, McKay RG, Baim DS, Keane JF. Transcatheter closure of ventricular septal defects. Circulation 1988;78:361–368.


4. Tofeig M, Patel RG, Walsh KP. Transcatheter closure of a mid-muscular ventricular septal defect with an amplatzer VSD occluder device. Heart 1999;81:438–440.



5. Thanopoulos BD, Tsaousis GS, Konstadopoulou GN, Zarayelyan AG. Transcatheter closure of muscular ventricular septal defects with the amplatzer ventricular septal defect occluder: initial clinical applications in children. J Am Coll Cardiol 1999;33:1395–1399.


6. Hijazi ZM, Hakim F, Al-Fadley F, Abdelhamid J, Cao QL. Transcatheter closure of single muscular ventricular septal defects using the amplatzer muscular VSD occluder: initial results and technical considerations. Catheter Cardiovasc Interv 2000;49:167–172.


7. Amin Z, Berry JM, Foker JE, Rocchini AP, Bass JL. Intraoperative closure of muscular ventricular septal defect in a canine model and application of the technique in a baby. J Thorac Cardiovasc Surg 1998;115:1374–1376.


8. Latiff HA, Alwi M, Kandhavel G, Samion H, Zambahari R. Transcatheter closure of multiple muscular ventricular septal defects using Gianturco coils. Ann Thorac Surg 1999;68:1400–1401.


9. Kalra GS, Verma PK, Singh S, Arora R. Transcatheter closure of ventricular septal defect using detachable steel coil. Heart 1999;82:395–396.



10. Pesonen E, Thilen U, Sandström S, Arheden H, Koul B, Olsson SE, et al. Transcatheter closure of post-infarction ventricular septal defect with the Amplatzer Septal Occluder device. Scand Cardiovasc J 2000;34:446–448.


11. Fedson S, Jolly N, Lang RM, Hijazi ZM. Percutaneous closure of a ruptured sinus of Valsalva aneurysm using the Amplatzer Duct Occluder. Catheter Cardiovasc Interv 2003;58:406–411.


12. Pedra CA, Pedra SR, Esteves CA, Pontes SC Jr, Braga SL, Arrieta SR, et al. Percutaneous closure of perimembranous ventricular septal defects with the Amplatzer device: technical and morphological considerations. Catheter Cardiovasc Interv 2004;61:403–410.


13. Michel-Behnke I, Le TP, Waldecker B, Akintuerk H, Valeske K, Schranz D. Percutaneous closure of congenital and acquired ventricular septal defects: considerations on selection of the occlusion device. J Interv Cardiol 2005;18:89–99.


14. Diab KA, Hijazi ZM, Cao QL, Bacha EA. A truly hybrid approach to perventricular closure of multiple muscular ventricular septal defects. J Thorac Cardiovasc Surg 2005;130:892–893.

15. Michel-Behnke I, Ewert P, Koch A, Bertram H, Emmel M, Fischer G, et al. Device closure of ventricular septal defects by hybrid procedures: a multicenter retrospective study. Catheter Cardiovasc Interv 2011;77:242–251.


16. Kirklin JK, Castaneda AR, Keane JF, Fellows KE, Norwood WI. Surgical management of multiple ventricular septal defects. J Thorac Cardiovasc Surg 1980;80:485–493.


17. Griffiths SP, Turi GK, Ellis K, Krongrad E, Swift LH, Gersony WM, et al. Muscular ventricular septal defects repaired with left ventriculotomy. Am J Cardiol 1981;48:877–886.


18. McDaniel N, Gutgesell HP, Nolan SP, Kron IL. Repair of large muscular ventricular septal defects in infants employing left ventriculotomy. Ann Thorac Surg 1989;47:593–594.


19. Wollenek G, Wyse R, Sullivan I, Elliott M, de Leval M, Stark J. Closure of muscular ventricular septal defects through a left ventriculotomy. Eur J Cardiothorac Surg 1996;10:595–598.


20. Kalra GS, Verma PK, Dhall A, Singh S, Arora R. Transcatheter device closure of ventricular septal defects: immediate results and intermediate-term follow-up. Am Heart J 1999;138(2 Pt 1): 339–344.


21. Amin Z, Gu X, Berry JM, Bass JL, Titus JL, Urness M, et al. New device for closure of muscular ventricular septal defects in a canine model. Circulation 1999;100:320–328.


22. Fishberger SB, Bridges ND, Keane JF, Hanley FL, Jonas RA, Mayer JE, et al. Intraoperative device closure of ventricular septal defects. Circulation 1993;88(5 Pt 2): II205–II209.

23. Chaturvedi RR, Shore DF, Yacoub M, Redington AN. Intraoperative apical ventricular septal defect closure using a modified Rashkind double umbrella. Heart 1996;76:367–369.



24. Murzi B, Bonanomi GL, Giusti S, Luisi VS, Bernabei M, Carminati M, et al. Surgical closure of muscular ventricular septal defects using double umbrella devices (intraoperative VSD device closure). Eur J Cardiothorac Surg 1997;12:450–454.


25. Bacha EA, Cao QL, Galantowicz ME, Cheatham JP, Fleishman CE, Weinstein SW, et al. Multicenter experience with perventricular device closure of muscular ventricular septal defects. Pediatr Cardiol 2005;26:169–175.


26. Lee EM, Roberts DH, Walsh KP. Transcatheter closure of a residual postmyocardial infarction ventricular septal defect with the Amplatzer septal occluder. Heart 1998;80:522–524.



27. Szkutnik M, Bialkowski J, Chodór P, Banaszak P, Poloński L, Nowak J, et al. Transcatheter closure of a residual postmyocardial infarction ventricular septal defect using Amplatzer atrial septal occluder. Folia Cardiol 2001;8:685–689.
28. Ruzyllo W, Demkow M, Konka M, Chmielak Z, Wilczynski J, Dzielinska Z, et al. Transcatheter closure of postinfarction ventricular septal defects. Kardiol Pol 2001;54:270–273.
29. Aitsiselmi A, Bonvini RF, Aggoun Y, Sigwart U. Percutaneous closure of a residual ventricular septal defect in a challenging patient. Acute Card Care 2006;8:21–24.


Fig. 1
Schematic of the procedure. RA, right atrium; LA, left atrium; RV, right ventricular; LV, left ventricle.
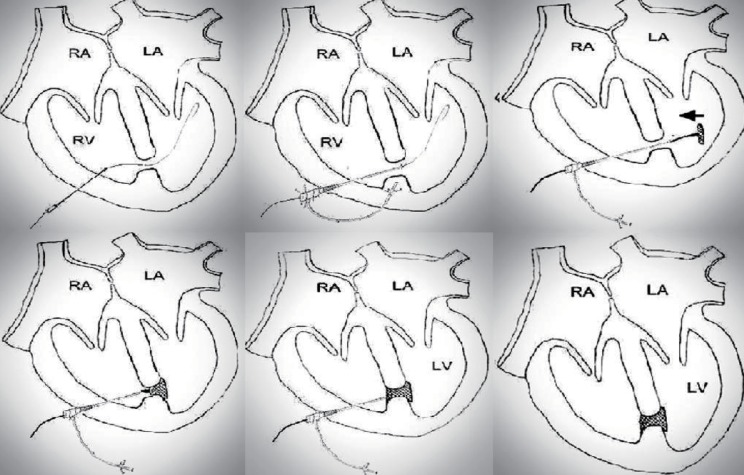
Fig. 2
Transesophageal echocardiography images of a 25-day-old boy with complete transposition of the great arteries and multiple muscular ventricular septal defects. The guide wire (A) and sheath (B) were passed through the ventricular septal defect perventricularly from the right ventricle to left venticle. A left-sided disc (aortic disc) was deployed (C) and pulled back to the septum (D) and a right-sided disc (pulmonic end) was configured (E). A minimal residual shunt was observed on color Doppler ultrasonography (F). On image D, a small amount of fluid collection is seen lateral to the left atrium.

Fig. 3
Echocardiographic images of procedural complications encounterd with patient 4. In the left ventricular cavity, the aortic disc appears to be entrapped within the left ventricular cavity wall (A). Left ventricular lateral wall damage and subsequent bleeding were visualized (B).
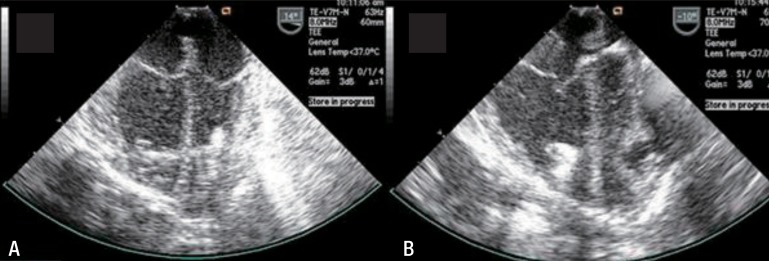
Fig. 4
Echocardiographic images of a pseudoaneurysm in patient 4. A postprocedure echocardiogram showed fluid collection at the left ventricular apex (arrow, A). An echocardiogram obtained 13 months later showed that the pseudoaneurysm had spontaneously regressed (arrow, B), and no leakage was seen on the left ventricular angiogram obtained at the same time point (C).
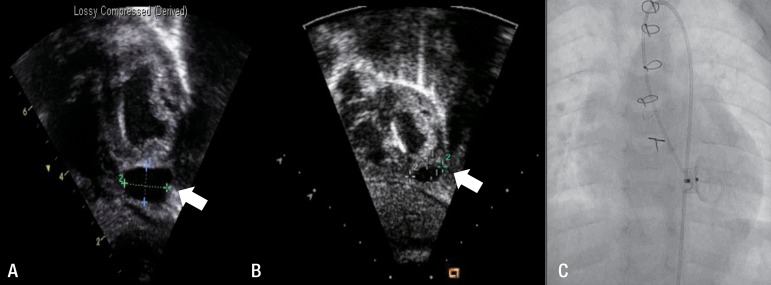
Table 1
Patient characteristics
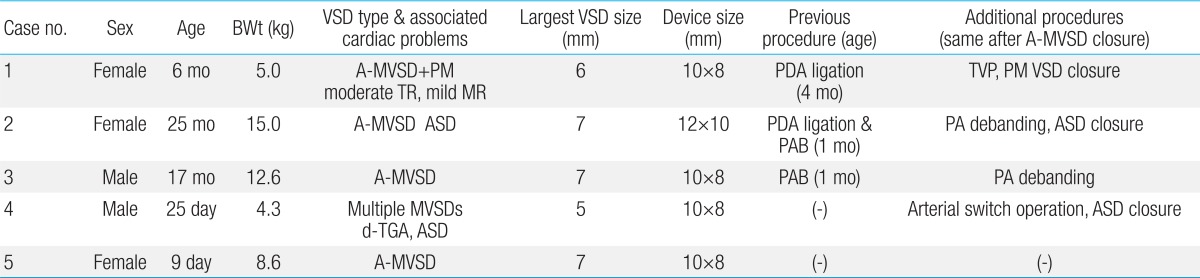
BWt, body weight; VSD, ventricular septal defect; A-MVSD, apical muscular ventricular septal defect; PM, perimembranous; TR, tricuspid regurgitation; MR, mitral regurgitation; PDA, patent ductus arteriosus; TVP, tricuspid valve plasty; ASD, atrial septal defect; PAB, banding of the pulmonary artery; PA, pulmonary artery; d-TGA, complete transposition of great arteries.



 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


