Article Contents
| Clin Exp Pediatr > Volume 67(10); 2024 |
|
Abstract
Two rehydration protocols currently exist to treat diabetic ketoacidosis (DKA) in pediatric patients aged <21 years: the traditional “one-bag” system and the more recent “two-bag” system. This study aimed to evaluate the safety and efficacy of the newer two-bag system versus the well-established one-bag system. The CiNAHL, Cochrane Library, Embase, PubMed, Scopus, and Web of Science databases were comprehensively searched from inception to June 2023 by 2 independent reviewers using the Preferred Reporting Items for Systematic Reviews and Meta-analysis framework. Eligible studies were those that reported participants <21 years of age who presented to the emergency room with a clinical diagnosis of DKA. This review was prospectively registered on PROSPERO (CRD42023427551). From the initial screening of 42 studies, 8 unique studies encompassing 583 patients met the eligibility criteria. The analysis yielded no significant intergroup differences in hypoglycemia (odds ratio, 0.61; 95% confidence interval [CI], 0.20–1.87; I2=3%) or mean glucose correction rate (mean difference [MD], 0.04 mg/ dL/hr; 95% CI, -13.10 to 13.17; I2=64%). The incidence of cerebral edema was as low (0.17%) across groups, with only one case reported in the one-bag group. Notably, the mean time to DKA resolution (MD, -3.24 h; 95% CI, -5.57 to -0.91; I2=0%) and mean response time for intravenous fluid changes (MD, -32.75 min; 95% CI, -43.21 to -22.29; I2=59%) was lower for the two-bag system. This meta- analysis presents preliminary evidence suggesting that the two-bag system may confer advantages over the one-bag system for selected patients. However, further studies with greater patient stratification based on DKA severity, fluid composition, and protocol are needed to draw definitive conclusions and elucidate the extent of these advantages.
Graphical abstract. KCl, potassium chloride; NS, normal saline; D5, 5% dextrose; D10, 10% dextrose; NS, normal saline; MD, mean difference; CI, confidence interval.
Diabetic ketoacidosis (DKA), often the initial manifestation of type 1 diabetes mellitus (T1DM), comprises 15%–70% of annual admissions to pediatric intensive care units (PICU) [1]. Furthermore, it remains prevalent among children with established T1DM, with an admission rate of 6%–8% per year [2]. It is important to note that DKA can also occur in patients with type 2 diabetes as well, although more commonly in adults than in children [3], and is being increasingly studied over time. Guidelines for DKA treatment do not discriminate between diabetes types. However, studies have suggested different treatment outcomes for DKA in type 2 versus type 1 diabetes in adults and children, such as longer time to hyperglycemia and acidosis resolution and insulin independence after DKA resolution [3,4].
The mainstay of DKA management is intravenous (IV) fluid resuscitation and insulin infusion aimed at restoring blood volume as well as glucose, electrolyte, and pH levels. However, fluid therapy may be associated with certain complications, most commonly cerebral edema.Its etiology remains unclear, but current theories postulate that it is correlated with rapid rehydration and subsequent decreases in osmolality along with other significant vasogenic factors [5,6]. Therefore, delicate interplay occurs between controlling the IV fluid administration rate to correct metabolic abnormalities and rapid rehydration, which may result in cerebral edema and hypoglycemia before acidosis resolution is achieved [7].
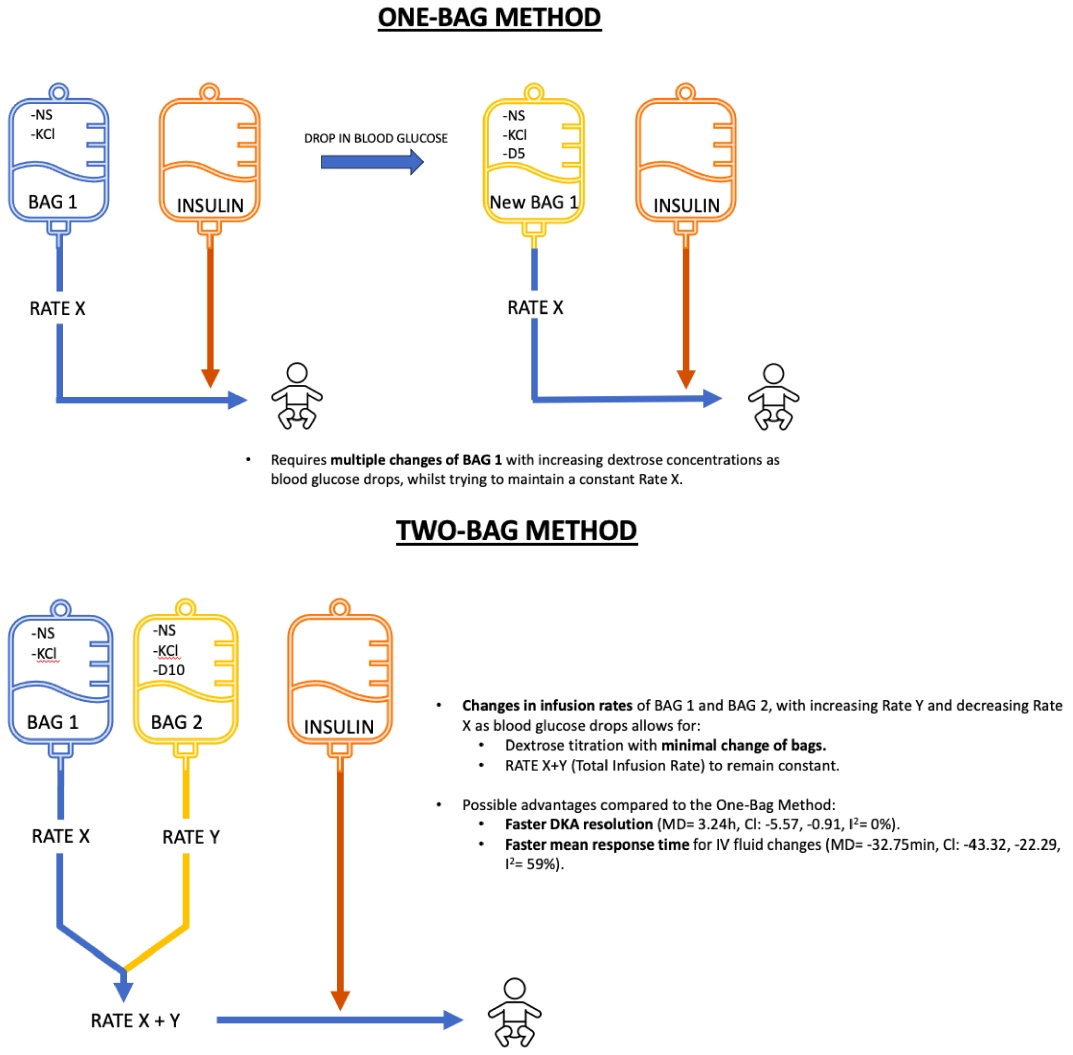
The traditional one-bag system used to treat DKA consists of one bag containing electrolytes administered alongside an IV insulin infusion with the aim of correcting the hypovolemia and hyperosmolality and resolving the acidosis. To prevent hypoglycemia, the bag was replaced with a new bag containing increasing concentrations of dextrose as the blood glucose levels declined. This system requires multiple fluid bag changes before their depletion. Consequently, this approach leads to a delayed response time for IV fluid changes, restricted variations of dextrose concentrations, and increased hospital costs [8,9].
In an attempt to overcome these limitations, the two-bag system was created at the Children’s Hospital of Philadelphia in 1999 [1,8]. This system consists of 2 bags of fluid administered simultaneously through a single IV line along with an IV insulin infusion. Bag 1 contains electrolytes, while bag 2 incorporates the same electrolyte composition plus dextrose. Independently controlling the infusion rates of each bag ensures a constant fluid infusion rate while tailoring the dextrose concentration to meet patient requirements.
Conflicting results were obtained by comparisons of the one- and two-bag protocols. Notably, some studies failed to identify any differences in the occurrence of hypoglycemic events [10], whereas others reported higher [11] or lower [12,13] frequencies of such events in the two-versus one-bag system. Some studies found no difference in the mean time to acidosis resolution [10], whereas others found that the two-bag system provided faster acidosis resolution [14,15]. These discrepancies also apply to other values such as the glucose reduction rate and average number of utilized fluid bags. Inevitably, a clear gap in the literature impedes the formulation of definitive guidelines for the optimal treatment regimens for pediatric DKA. In aspiring to address this, here we present the first systematic review and meta-analysis to evaluate the current literature on the safety and efficacy of the two- versus one-bag system in patients <21 years of age.
This review adhered to the Preferred Reporting Items for Systematic Reviews and Meta analyses (PRISMA) guidelines [16]. We conducted a comprehensive search of the CiNAHL, Cochrane Library, Embase, PubMed, Scopus, and Web of Science databases from their inception to June 1, 2023. The search was designed and conducted using a qualified medical reference library. Controlled vocabulary supplemented with keywords was used to search for studies describing DKA, one-bag and two-bag systems, and pediatric populations. The actual strategy, including the search terms used and how they were combined, is shown in Supplementary material 1. This review was prospectively registered on PROSPERO (CRD42023427551).
Eligible studies met all of the following inclusion criteria: (1) age ≤21 years and presenting to the hospital with DKA symptoms; (2) clinical diagnosis of DKA; and (3) findings related to at least one of the primary outcomes of hypoglycemia, mean glucose correction rate, and cerebral edema. Individuals aged ≤21 years were designated pediatric subjects in accordance with American Academy of Pediatrics guidelines [17].
Case reports, case series, and review articles were also excluded. Studies in which patients had cerebral edema at presentation, received insulin prior to admission, or received prior steroid therapy were also excluded. Two authors (MLN and RBZ) independently evaluated the methodological quality of each study using the Newcastle-Ottawa Scale [18]. Discrepancies were resolved by using an adjudicator (NY, USA). The methodology and results of the quality assessment of all included studies are shown in Supplementary Table 1.
The outcomes of interest for this meta-analysis included the incidence of hypoglycemia and cerebral edema along with the mean glucose correction rate. Other pertinent outcomes included the average number of IV fluid bags used, mean time to DKA resolution, mean bicarbonate correction rate, mean PICU length of stay (LOS), and mean response time to IV fluid changes using odds ratios (ORs) and mean differences (MDs).
The International Society for Pediatric and Adolescent Diabetes defines DKA as the presence of hyperglycemia (blood glucose >200 mg/dL), metabolic acidosis (venous pH <7.3, serum bicarbonate <15 mEq/L), and ketosis (>3 mmol/L β-hydroxybutyrate) in the blood or “moderate or large” urine ketones) [2]. The patients in all 8 studies met the diagnostic criteria for DKA [8,10-15,19].
The pooled means and proportions were analyzed using the inverse variance method for continuous data and the Mantel-Haenszel method for dichotomous data. These methods assign weights to each study based on its variance. To directly compare the 2 treatment systems, we conducted a two-arm analysis of studies that reported the outcomes of both treatments. The heterogeneity of the effect size estimates across studies was quantified using the Q statistic and I2. An I2 value of 0%–25% indicates negligible statistical heterogeneity, 26%–50% denotes low heterogeneity, and 51%–100% indicates high heterogeneity [21]. A random-efects model was used when the value of I2 was >50%, whereas a fixed-effects model was used for values of I2 <50%. Publication bias was assessed using a funnel plot [22]. If mean and standard deviation (SD) data were unavailable, the median was converted to the mean using formulas from the Cochrane Handbook for Systematic Reviews of Interventions [23]. The data analysis was performed using RevMan software version 5.4 (The Cochrane Collaboration, Copenhagen, Denmark).
The initial search yielded 42 potentially relevant articles, of which 8 unique studies involving 583 patients met the eligibility criteria [8,10-15,19]. The study selection process and PRISMA flow diagram are depicted in Supplementary Fig. 1.
The outcomes of the quality assessment of all the included studies are shown in Supplementary Table 1. Five studies [8,10-12,14] were judged as being of good quality. The patients represented the entire experience of the investigator. The exposure and outcomes were adequately ascertained, and the follow-up was adequate. Three studies were judged as being of poor quality, as follow-up length and adequacy were unclear [15,19] or intergroup comparability was not established [13]. Nonetheless, all of the included studies were deemed adequate within the selection domain.
The baseline and clinical characteristics of the included studies are summarized in Table 1.Within the scope of the 8 studies, 583patients (52.1% female) with a mean age ranging from 6.67 to 15.6 years old collectively contributed to at least 852 DKA episodes. However, the exact total number of DKA episodes remains unclear because Grimberg et al. [8] referred to repeated episodes without specifying the overall count, which led to ambiguity in the cumulative total. Wolfgram et al. [13] presented their results in the context of DKA. The remaining studies reported outcomes based on the number of patients, which was equivalent to the number of episodes.
Of the 295 patients for whom classification in each group was specified, 145 (49.2%) received one-bag treatment and 150 (50.8%) received two-bag treatment. An analysis of the sex distribution revealed that the one-bag group was 59.7% female (83 of 139), while the two-bag group was 57.5% female (83 of 144) [8,10-12,15,19]. At least 237 DKA episodes (27 %) were managed using the one-bag system, while at least 615 episodes (72.2%) were managed using the two-bag system [10-15,19].
Furthermore,the mean weights were similar between the one- and two-bag groups (48.67±16.14 kg and 48.62±16.45 kg, respectively) [8,11,15,19]. The majority of patients had an established diagnosis of diabetes mellitus: 66.2% (96 of 145) in the one-bag group versus 64.7% (97 of 150)in the two-bag group [8,10-12,15].
Across all studies, the IV fluid in the one-bag group consisted of sodium chloride (NaCl) at various concentrations, most commonly 0.9% NaCl [8,11,15] and 0.45% NaCl [10,12,15,19], alongside lactated Ringer solution [15], potassium derived from potassium chloride (KCl) [8,11,12,14,15], potassium phosphate (KPO4) [8,11,12,15,19], and/or potassium acetate (Kacetate) [15,19]. Glucose (5% or 10% dextrose) was added when the blood glucose fell below a certain level. In the two-bag group, the composition of the first bag mirrored that of the one-bag group without additional glucose. The second bag had the same composition with added glucose, predominantly 10% dextrose [8,11-15,19], except in the study of Dhochak et al. [10], which used 12% dextrose.
The mean duration of IV fluid infusion ranged from 16.6 to 31.5 hours in the two-bag group and from 17.8 to 31.7 hours in the one-bag group (Table 2). A comprehensive analysis of the forest plot showed no intergroup difference (Supplementary Fig. 2). The mean initial insulin drip rate was set between 0.05 and 0.10 units/kg/hr in both groups in most studies. However, each study used a different protocol, particularly regarding the blood glucose modification thresholds of the 2 bags. The initiation threshold for the bag containing glucose ranged from 300 to 400 mg/dL, whereas the threshold at which this glucose-containing bag constituted 100% of the total fluid delivery ranged from 150 to 225 mg/dL. One study used a specific protocol, such as the subcutaneous delivery of insulin after fluid administration instead of a simultaneous infusion [14]. Moreover, some studies did not follow a specific protocol for glucose titration and/or fluid composition [8,15,19], whereas others used well-defined protocols [10-14]. Details of the IV fluid composition and protocol overview for each bag system in each study are described in Table 2.
The initial biochemical values of the patients in the included studies are summarized in Table 3. In one of the studies [15], severe DKA was defined as a pH <7.10 mEq/L or bicarbonate <5.00 mEq/L, while moderate DKA was defined as a pH <7.20 mEq/L or bicarbonate <10.00 mEq/L. Overall, the mean pH ranged from 7.07 mEq/L to 7.20 mEq/L in the one-bag groups and from 7.04 mEq/L to 7.17 mEq/L in the two-bag groups. One study excluded patients with severe DKA based on the inclusion criteria [14].
Bicarbonate levels exhibited a mean range of 7.20 mEq/L to 10.76 mEq/L in the one-bag groups and 6.30 mEq/L to 10.60mEq/Linthe two-bag groups.Glucose levels displayed a mean range of 435.20 mg/dL to 568.00 mg/dL in the onebag groups and 455.00 mg/dL to 629.90 mg/dL in the two-bag groups. The mean glucose levels were slightly higher in the two-bag groups. Correspondingly, there appears to be a general trend of bicarbonate and pH values being lower in the two-bag group, but the overall DKA severity seemed similar between groups and across studies [10-12,15,19].
The outcomes of the different studies were reported in Table 4.
It is essential to acknowledge the variability in the definitions of hypoglycemia used in different studies. The distinct blood glucose thresholds for categorizing hypoglycemia across different studies include 50 mg/dL [10], 60 mg/dL [11], and 70 mg/dL [12,13], the latter being in accordance with the definition recognized by the Internal Society for Pediatric and Adolescent Diabetes [20].
Four studies [10-13] reported the incidence of hypoglycemia with a total of 560 DKA episodes in the two-bag group versus 196 in the one-bag group.Additionally, 3 studies [11,15,19] reported the mean glucose correction rate, with 96 patients in the two-bag group and 77 patients in the one-bag group. The pooled estimates revealed no significant differences in hypoglycemia incidence (OR, 0.61; 95% CI, 0.20–1.87; I2=73%) or the mean glucose correction rate between the 2 groups (MD, 0.04 mg/dL/hr; 95% CI, -13.10 to 13.17; I2=64%) (Fig. 1). Cerebral edema, with a very low incidence rate of 0.17%, was examined in all 6 studies that reported complications [8,10-12,14,19]. Notably, this occurred in a single patient in the one-bag group [11]. Cerebral edema was defined as a Glasgow Coma Scale score of<14 coupled with computed tomography findings. The patient was treated with hypertonic saline and achieved a complete neurological recovery [11].
Three studies [8,11,19] reported the average number of fluid bags used, with a total of 84 patients in the two-bag group versus 78 in the one-bag group. Additionally, 2 studies [11,12] reported on the PICU LOS with a total of 80 patients in the two-bag group and 90 patients in the one-bag group. No significant intergroup differences were observed in the number of fluid bags used (MD, -0.82 bags; 95% CI, -3.31 to 1.67; I2=98%) or PICU LOS (MD, -0.29 days; 95% CI, -1.20 to 0.61; I2=94%) (Fig. 2A and B). Conversely, 2 studies [8,19] reported the mean response time for IV fluid changes of 27 patients in the two-bag group and 26 patients in the one-bag group. These findings revealed that the mean response time for IV fluid changes was longer in the two-bag than one-bag group (MD, -32.75 minutes; 95% CI, -43.21 to -22.29;I2=59%) (Fig. 2C).
Efficacy outcomes also include the resolution of biochemical parameters. Two studies [15,19] reported the mean bicarbonate correction rate of 39 patients in the two-bag group and 25 patients in the one-bag group. Furthermore, 2 studies [12,15] reported the mean time to pH resolution, with 45 patients in the two-bag group and 47 patients in the one-bag group. No significant intergroup differences were seen in bicarbonate correction rate (MD, 0.25 mEq/L/hr; 95% CI, -0.02 to 0.51; I2=45%) or mean time to pH resolution (MD, -0.33 hour; 95% CI, -2.72 to 2.06; I2=0%) (Fig. 2D and E).
Two studies [10,14] provided data on the mean±SD time to DKA resolution of 21 patients in the two-bag group and 20 patients in the one-bag group. DKA resolution was defined as bicarbonate level, pH, and/or glycemia correction. Mean time to DKA resolution was faster in the two-bag than one-bag group (MD, -3.24 minutes; 95% CI, -5.57 to -0.91; I2=0%) (Fig. 2F).
Controversy persists about whether the one- or two-bag system is the optimal management plan for pediatric DKA. This study, the first meta-analysis to examine the existing literature, aimed to provide preliminary insights that may contribute to the ongoing discourse. Our findings demonstrate that the two-bag system decreases the DKAresolution time and the mean response time to IV fluid changes. The 2 protocols exhibited similar hypoglycemia and mean glucose correction rates and a very low cerebral edema rate, with only one case reported across all included studies. Similarly, the pH and bicarbonate resolution times and rates, as well as the PICU LOS, did not differ significantly between the 2 groups.
Continuing insulin therapy beyond the achievement of normoglycemia, often due to persistent acidosis, may precipitate hypoglycemia. When severe, this complication can result in convulsions, loss of consciousness, and transient neurological deficits, and it constitutes 4%–10% of cases of mortality among patients with T1DM [24]. Consequently, the adequate addition of glucose to IV fluids is a crucial component of DKA management. Across 4 studies, our results showed no difference in hypoglycemia between the 2 bag systems [8,11-13], with 2 studies reporting no incidence of hypoglycemia [8,14]. However, some factors related to patients themselves may play a larger role in the development of hypoglycemia than the management type used. For instance, in 1 study, the incidence of hypoglycemia was the same for different bag systems but higher among malnourished children regardless of treatment group [10]. These insights underline the multifactorial nature of hypoglycemic risk in the management of DKA.
Moreover, the two-bag system allows for faster glucose titration [6,12]; however, if this leads to inadequate blood glucose monitoring [11], the potential benefits of the two-bag system may be compromised. Therefore, to achieve a faster glucose titration and prevent hypoglycemia, the application of standardized DKA guidelines is crucial. Thus, all aspects of treatment, not just the fluid delivery method, may be optimized. This hypothesis was supported by Wolfgram’s quality improvement initiative, which included a total of 557 visits. DKA management was standardized in terms of treatment protocols, inclusion and education of all stakeholders, handoffs between the Emergency Department and inpatient registered nurses, and explicit electronic medical record orders. These quality improvements result in lower hypoglycemia rates among patients treated with the two-bag system [13]. Our meta-analysis revealed no significant intergroup difference in the glucose correction rate. This may partly explain the lack of intergroup disparity in the hypoglycemic rates. The glucose correction rate and hypoglycemia are related; a slower glucose correction rate provides more time to adjust fluid glucose delivery based on blood glucose levels, which would aid in the prevention of hypoglycemia. Conversely, a faster glucose correction rate may be more challenging to control and potentially precipitate hypoglycemia. Therefore, the glucose correction rate is typically limited to <100mg/dL/hr [15,19]. In all 3 studies reporting this parameter [11,15,19], the glucose correction rate was appreciably below the 100 mg/dL/hr threshold in both groups, showing effective control regardless of the bag system used. However, it is worth acknowledging that these 3 studies had small sample sizes, which may have impacted the results.
Children are particularly susceptible to cerebral edema due to ongoing central nervous system maturation [12]. Across all included studies [11,15,19], only one case of cerebral edema was reported in the one-bag group; this incident was effectively treated without long-term sequelae [11]. Although rare, comprising only 1% of pediatric DKA episodes, cerebral edema is associated with significant mortality, accounting for 57%–87% of all DKA-related deaths. Its signs and symptoms include headache, decreased consciousness, bradycardia, hypertension, and increased intracranial pressure [5,6]. As previously mentioned, its precise etiology remains unclear; however, given that osmotic factors may play a role, controlling fluctuations in serum glucose concentrations is of paramount importance. It is important to note that both groups in the 3 included studies maintained adequate control of serum glucose concentrations [11,15,19]. Moreover, elevated blood urea nitrogen levels and marked hypocapnia at presentation are potential risk factors; however, these factors have not been reported in patients who develop cerebral edema [5,6].
In terms of fluid-related variables, no difference in the number of fluid bags used was observed between the two-and one-bag systems. This finding was consistent with studies conducted by Hasan et al. [11] and Poirier et al. [19], who also reported a low average of 3–4 fluid bags. This implies that a highly optimized IV fluid implementation may have a more pronounced effect on the number of bags used than the choice of bag system. The mean response time from the physician’s order to the implementation of IV fluid changes was shorter in the two- versus one-bag system. For adjustments in the two-bag group, this response time was nearly instantaneous in 1 study [19] and up to 7 min in another [8], whereas in the one-bag group, the response time exceeded 30 minutes in both studies [8,19]. This increased efficiency of healthcare staff enables rapid adjustments and reduces large fluctuations in fluid status. However, given the small sample size of the current meta-analysis, it is essential to highlight the need for further research to increase our understanding.
In terms of biochemical parameters, the mean time to DKA resolution, which incorporated bicarbonate, blood glucose, and venous pH,was shorter for the two- versus onebag system. This observation is consistent with the results of the study of Ferreira et al. [14]. However, this finding was inconsistent with the results of Dhochak et al. [10]. Moreover, no intergroup difference was observed in the time to resolution of acidosis or bicarbonate correction rate when assessed separately. This could indicate an overall greater efficacy of the two-bag system. However, the conflicting outcomes mentioned above may be attributed to the fact that patients were not stratified based on DKA severity at presentation. Recent studies indicated that higher glucose and lower pH levels are independent predictors of longer time to resolution [25]. Therefore, it is imperative that DKA severity be considered in future investigations to reduce confounders. Another significant explanation of why DKA resolution was lower in the two-bag group but no differences were found when pH resolution time and bicarbonate correction rate were assessed separately is that the studies that reported on DKA resolution [10,14] differed from those that reported on pH [12,15] and bicarbonate [15,19] resolution. The need for future studies to report similar outcomes, as well as suggestions for important outcomes, are further discussed below.
As with all meta-analyses, this study has limitations that should be acknowledged. Notably, different quantifiers were used to define DKA resolution: One study used all 3 criteria (pH ≥7.3, bicarbonate ≥15 mEq/L, and glycemia ≤250 mg/dL [14]), another study used only pH and bicarbonate [10], and 2 studies relied solely on pH [12,15]. Interestingly, only 1 study measured ketone resolution time [15]. Further studies should ideally report on all 3 parameters to ensure a more precise evaluation of DKA resolution given that the definition of DKA expressed by the International Society for Pediatric and Adolescent Diabetes includes meeting the criteria for all 3 parameters [2]. Furthermore, although the overall DKA severity seemed similar between groups and across studies [10-12,15,19], it would be more beneficial to compare the mean time to DKA resolution between the 2 groups for different severities to minimize potential confounding factors. Furthermore, urine ketones and plasma osmolality, which were reported in only 1 study [10], could be useful for stratifying DKA severity along with pH, bicarbonate, and glucose parameters. To further eliminate confounding factors and improve study quality (Supplementary Table 1), it was essential to delineate the baseline characteristics of the one- versus two-bag groups for each DKA episode. It could also be useful if future studies report body weight in terms of age- and sex-adjusted SD scores of body weight to ensure that body weight is truly similar between groups and does not act as a potential confounding factor.
As illustrated in Table 3, each study employed a different protocol in terms of fluid composition, insulin administration mode and timing, and glucose titration guidelines. Some studies provided detailed titration protocols based on blood glucose concentrations [10-13], whereas others did not [8,15,19]. Future studies comparing the one- and two-bag systems should establish clear protocols to ensure standardized treatment across all patients and eliminate confounders. The significance of standardized management protocols was demonstrated in the study of Wolfgram et al. [13] as well as in the study of Babbitt et al. [1], in which implementing an IV fluid titration algorithm resulted in faster blood glucose resolution and maintenance for patients treated with the two-bag fluid system.
Additionally, the forest plots displayed high heterogeneity (I2>50%) for the 5 outcomes of hypoglycemia, mean glucose correction rate, mean response time for IV fluid bag changes, average number of fluid bags used, and mean PICU LOS, with a heterogeneity of I2>90% for the latter two. This high heterogeneity further supports the idea that confounders such as DKA severity and differences within protocols may have influenced the results.
Other potential confounders include comorbidities such as malnutrition and pre-existing T1DM. Conversely, the heterogeneity rate was 0% for time to DKA resolution and time to pH resolution, which may indicate greater reliability. However, the extent to which conclusions can be drawn from the results remains unclear. For most outcomes, the arguments presented here are preliminary in nature owing to small sample sizes and the limited number of studies reporting each outcome.
This raises an important question: What are the most reliable indicators of safety and efficacy of IV fluids used to treat DKA in pediatric patients? A consensus on preferred outcomes should be reached to encourage future studies to report consistent measures. Moreover, most of the included studies were retrospective with only 2 randomized controlled trials. This introduces the risk of selection bias. Ideally, future meta-analyses should include more randomized controlled trials with similar outcomes and larger sample sizes to provide more accurate evidence.An example of such outcomes could be the levels of β-hydroxybutyrate (BOHB), the primary ketone body in DKA. Interestingly, none of the studies included in this meta-analysis incorporated BOHB concentration into their DKA management protocols. Previous studies demonstrated that regular bedside serum BOHB concentrations is a valuable tool for monitoring treatment response and establishing criteria for assessing DKA resolution. In future studies of DKA resolution, the inclusion of BOHB measurements should be considered because of the benefits they offer for monitoring patients through serial measurements [26]. It is also important for studies to adopt similar definitions for outcomes such as hypoglycemia that align with recent international guidelines such as those provided by the International Society for Pediatric and Adolescent Diabetes [20].
Despite the limitations and preliminary nature of this meta-analysis, its principal objective was to serve as an important first step in elucidating where current evidence stands on the safety and efficacy of the two-bag versus traditional one-bag system in patients <21 years of age with DKA. This study also offers guidance for future research endeavors seeking to optimize DKA management protocols and outcomes.
The preliminary results of the current meta-analysis suggest that the two-bag system could be an alternative to the one-bag system for the initial treatment of DKA in selected patients <21 years of age. This was dictated by our observations, which revealed no discernible differences between systems in hypoglycemia, glucose correction rates, or the occurrence of cerebral edema. The two-bag system showed a faster mean time to DKA resolution and a faster mean response time for IV fluid changes, implying potential advantages in terms of efficacy. In terms of cost-effectiveness, no significant intergroup differences were observed in the average number of fluid bags. However, further studies are required that feature greater patient stratification based on DKA severity, fluid composition, insulin administration timing, and glucose titration protocols. These efforts will further elucidate a more comprehensive understanding of the comparative efficacy of the two- versus one-bag system for DKA treatment.
Supplementary materials
Supplementary material 1, Table 1, and Figs. 1 and 2 can be found via https://doi.org/10.3345/cep.2023.01536.
Supplementary Fig. 1. Study selection process and PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) flow diagram.
cep-2023-01536-Supplementary-Fig-1.pdf
Supplementary Fig. 2. Forest plot showing mean duration of IV fluid infusion. SD, standard deviation; IV, inverse variance; CI, confidence interval; df, degree of freedom.
cep-2023-01536-Supplementary-Fig-2.pdf
Acknowledgments
The authors thank Marcos Riba of the University of Queensland for conducting the literature search.
Fig. 1.
Forest plots of the comparison of hypoglycemia (A) and mean glucose correction rates (B) between the two- and one-bag groups through a random-effects model. M-H, Mantel-Haenszel; CI, confidence interval; df, degrees of freedom; IV, inverse variance; SD, standard deviation.
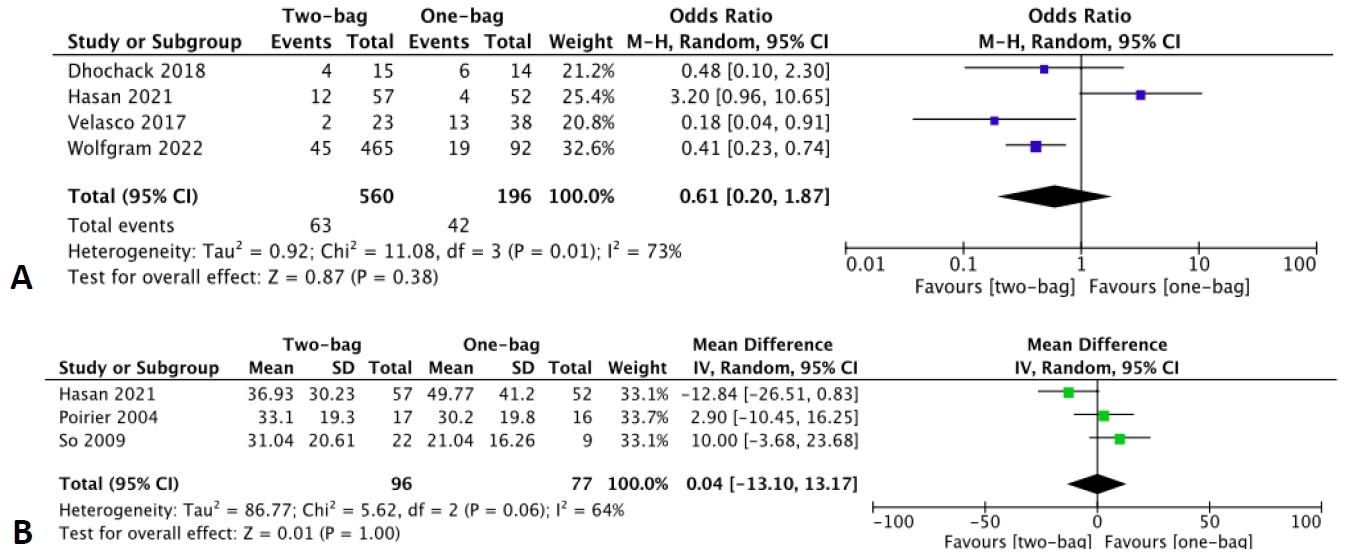
Fig. 2.
Forest plots of the comparison of average number of fluid bags used (A), pediatric intensive care unit length of stay (B), mean response time for intravenous fluid changes (C), mean bicarbonate correction rate (D), mean time to pH resolution (E), and mean time to diabetic ketoacidosis resolution (F) between the two- and one-bag groups through random (A, B, C) and fixed (D, E, F) effects models. CI, confidence interval; df, degrees of freedom; IV, inverse variance; SD, standard deviation.
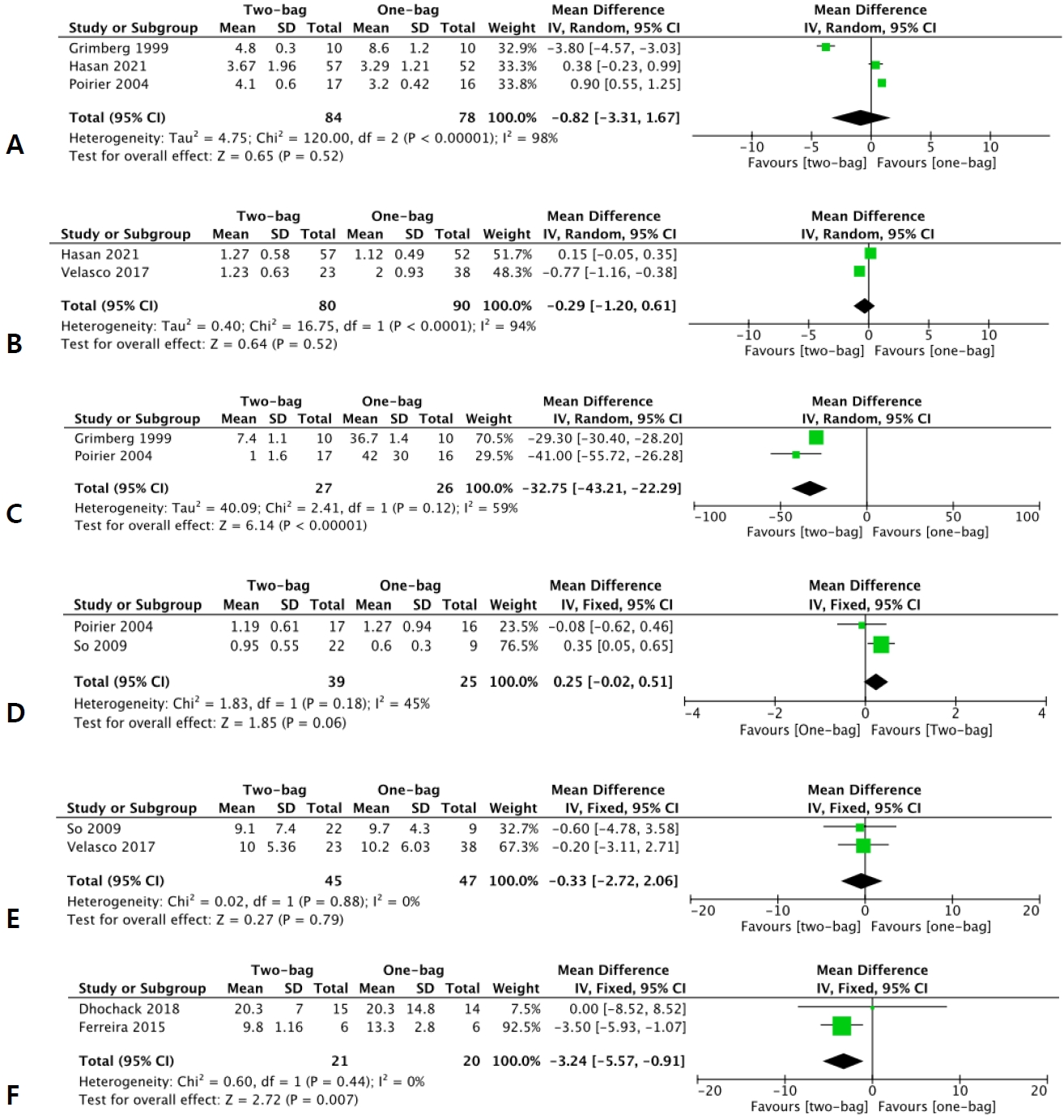
Table 1.
Baseline and clinical characteristics
| Study | Country | Study design | Combined (N) | One-bag (1B) (N) | Two-bag (2B) (N) | Age (yr), mean±SD | Weight (kg), mean±SD | Established diabetes mellitus type 1 (N) |
|---|---|---|---|---|---|---|---|---|
| Dhochak et al., [10] 2018 | North India | Prospective open labelled randomized controlled pilot trial | Total: 29 | Total: 14 | Total: 15 | Total: mean, 6.67 | 1B: NR | 1B: 5 |
| Female: 13 | Female: 5 | Female: 8 | 1B: mean, 6.34 | 2B: NR | 2B: 6 | |||
| 2B: mean, 6.90 | ||||||||
| Ferreira et al., [14] 2015 | Argentina | Randomized controlled trial | Total: 12 | Total: 6 | Total: 6 | Total: 9.30±3.40 | 1B: NR | 1B: NR |
| Female: 10 | Female: NR | Female: NR | 1B: NR | 2B: NR | 2B: NR | |||
| 2B: NR | ||||||||
| Grimberg et al., [8] 1999 | USA | Case-controlled retrospective analysis | Total: 20 | Total: 10 | Total: 10 | Total: 12.75±2.19 | 1B: 38.40±9.90 | 1B: 6 |
| Female: 14 | Female: 6 | Female: 8 | 1B: 12.00±2.00 | 2B: 48.50±13.30 | 2B: 6 | |||
| 2B: 13.50±2.20 | ||||||||
| Hasan et al., [11] 2021 | USA | Retrospective cohort study | Total: 109 | Total: 52 | Total: 57 | Total: 13.24±3.90 | 1B: 48.86±17.12 | 1B: 45 |
| Female: 68 | Female: 34 | Female: 34 | 1B: 12.96±4.78 | 2B: 50.90±16.25 | 2B: 47 | |||
| 2B: 13.50±2.91 | ||||||||
| Poirier et al., [19] 2004 | USA | Prospective study | Total: 33 | Total: 16 | Total: 17 | Total: 12.61±3.93 | 1B: 50.70±13.00 | 1B: NR |
| Female: 18 | Female: 9 | Female: 9 | 1B: 14.10±2.20 | 2B: 39.90±17.30 | 2B: NR | |||
| 2B: 11.20±4.70 | ||||||||
| So et al., [15] 2009 | USA | Retrospective study | Total: 31 | Total: 9 | Total: 22 | Total: 13.26±3.94 | 1B: 55.40±17.70 | 1B: 6 |
| Female: 19 | Female: 7 | Female: 12 | 1B: 13.90±3.00 | 2B: 49.50±16.40 | 2B: 16 | |||
| 2B: 13.00 ±4.30 | ||||||||
| Velasco et al., [12] 2017 | USA | Retrospective study | Total: 61 | Total: 38 | Total: 23 | Total: 15.60±3.79 | 1B: NR | 1B: 34 |
| Female: 28 | Female: 22 | Female: 12 | 1B: 15.20±3.94 | 2B: NR | 2B: 22 | |||
| 2B: 16.20±3.50 | ||||||||
| Wolfgram et al., [13] 2022 | USA | Plan-do-study-act cycle | Total: 288 | Total: NR | Total: NR | Total: 13.10±3.70 | 1B: NR | 1B: NR |
| Female: 134 | Female: NR | Female: NR | 1B: NR | 2B: NR | 2B: NR | |||
| 2B: NR |
Table 2.
Treatment details
| Study | IV fluid composition (1B) | IV fluid composition (2B) | Duration of IV fluid infusion (hr), mean±SD | Initial insulin drip rate (units/kg/hr), mean±SD | Protocol main points (1B) | Protocol main points (2B) |
|---|---|---|---|---|---|---|
| Dhochak et al., [10] 2018 | NaCl (77 mEq/L), K+ (40 mEq/L) | 1B: NaCl (77 mEq/L), K+ (40 mEq/L) | 1B: NR | 1B: 0.10±0.00 | Dextrose concentration in the IV fluid was changed in steps of 2.5% to maintain BG values within 150–200 mg/dL. | Dextrose concentration in the IV fluid was titrated in steps of 2.5% by increasing the rate of 2B while simultaneously decreasing the rate of 1B by the same amount to maintain BG values within 150–200 mg/dL |
| Dextrose 5% added when glucose <250 mg/dL | 2B: same+dextrose 12.5% | 2B: NR | 2B: 0.10±0.00 | |||
| Ferreira et al., [14] 2015 | Glucos e, Na C l (70 mEq/L then 50 mEq/L), KCl (30 mEq/L then 40 mEq/L) | 1B: NaCl, KCl | 1B: mean, 24 | 1B: NA (SC insulin) | First 6 hr: 2,100 mL/m2 with initial glucose flow of 3.5 mg/kg/min and 70 mEq/L of NaCl and 30 mEq/L of KCl | First 6 hr: 2,100 mL/m2 with initial glucose flow of 3.5 mg/kg/min and 70 mEq/L of NaCl and 30 mEq/L of KCl |
| 2B: same+10% dextrose | 2B: mean, 24 | 2B: NA (SC insulin) | Following 18 hr: 2,100 mL/m2 with initial glucose flow of 3.25 mg/kg/min and 50 mEq/L of NaCl and 40 mEq/L of KCl | Following 18 hr: 2,100 mL/m2 with initial glucose flow of 3.25 mg/kg/min and 50 mEq/L of NaCl and 40 mEq/L of KCl | ||
| SC insulin instead of infusion on an hourly basis after fluid resuscitation, depending on serum glucose | SC insulin instead of infusion on an hourly basis after fluid resuscitation, depending on serum glucose | |||||
| Grimberg et al., [8] 1999 | NaCl, KCl, KPO4 | 1B: NaCl, KCl, KPO4 | 1B: 17.80±7.50 | 1B: mean, 0.10 | NR | NR |
| Glucose when needed | 2B: same+D10 | 2B: 16.60±5.00 | 2B: mean, 0.10 | |||
| Hasan et al., [11] 2021 | 0.9% NaCl, 20 mEq/L KCl or 15 mMol/L KPO4 | 1B: 0.9%NaCl, 20 mEq/L KCl or 15 mMol/L KPO4 | 1B: NR | 1B: 0.10±0.00 | Start with isotonic saline over 1 hr for all patients | Start with isotonic saline over 1 hr for all patients |
| Dextrose when glucose <300 mg/dL | 2B: same+10% dextrose | 2B: NR | 2B: 0.10±0.00 | When BG > 300 mg/dL, 100% of the prescribed IV fluid was delivered from the bag that did not contain dextrose | ||
| When BG <150 mg/dL: 100% fluid delivered from the bag with D10 | ||||||
| Poirier et al., [19] 2004 | 1/2 NaCl, Kacetate +/- KPO4, D10 | 1B: 1/2 NaCl, Kacetate +/- KPO4 | 1B: NR | 1B: mean, 0.10 | Initial resuscitation: NS 10–20 mL/kg over 1 hr | Initial resuscitation: NS 10–20 mL/kg over 1 hr |
| 2B: same + D10 | 2B: NR | 2B: mean, 0.10 | Rehydration fluids: begin to add dextrose to the fluid as BG approaches 350–400 mg/dL | |||
| So et al., [15] 2009 | 0.9% NaCl or lactate Ringer or 0.45% NaCl | 1B: NaCl (50%) or lactate Ringer (41%)+potassium (KPO4 or KCl or KAcetate) | 1B: 31.70±17.40 | 1B: 0.05 (±0.00) | NR | NR |
| Added D5 when needed+potassium (KCl, KPO4 or K acetate) | 2B: D10, 1/2NS (55%) or D10, NS (36%)+potassium (KPO4 or KCl or KAcetate) | 2B: 31.50±18.60 | 2B: 0.07 (±0.02) | |||
| Velasco et al., [12] 2017 | 1⁄2 NS+ 20 mEq/L KCl+20 mmol/L KPO4 | 1B: 1/2 NS or NS | 1B: NR | 1B: 0.05 (±0) | NR | NS bolus from 10 ml/kg to 20 mL/kg. |
| D5 added once BG <300 mg/dL (at same rate) | 2B: D10, 1/2 NS or D10, NS | 2B: NR | 2B: 0.05 (±0) | When BG>300 mg/dL, fluid is delivered entirely by 1B. When BG<225 mg/dL, fluid is delivered entirely by 2B | ||
| Wolfgram et al., [13] 2022 | NR | 2 Bags with identical electrolyte concentrations, but one IV fluid bag contained no dextrose while the other bag contained dextrose 10% (D10). | 1B: NR | 1B: NR | NS bolus from 10 mL/kg to 20 mL/kg | When BG<300 mg/dl, change rate to 50% for each bag. |
| 2B: NR | 2B: NR | When BG <150 mg/dL, deliver 100% of the fluid via the bag with D10 | ||||
| Turn insulin drip off if blood glucose falls below 100 |
IV, intravenous; 1B, one-bag group; 2B, two-bag group; SD, standard deviation; NR, not reported; BG, blood glucose; NaCl, sodium chloride; K+, potassium; KCl, potassium chloride; NA, not applicable; SC, subcutaneous; KPO4, potassium phosphate; NS, normal saline; D5, 5% dextrose; D10, 10% dextrose; 1/2NS, half normal saline.
Table 3.
Initial biochemical values
| Study | Glucose (mg/dL), mean±SD | Bicarbonate (mEq/L), mean±SD | pH (mEq/L), mean±SD |
|---|---|---|---|
| Dhochak et al., [10] 2018 | 1B: 440.0±74.0 | 1B: 7.50±5.40 | 1B: 7.09±0.17 |
| 2B: 455.0±47.0 | 2B: 6.30±2.60 | 2B: 7.04±0.11 | |
| Ferreira et al., [14] 2015 | 1B: NR | 1B: NR | 1B: NR |
| 2B: NR | 2B: NR | 2B: NR | |
| Grimberg et al., [8] 1999 | 1B: NR | 1B: NR | 1B: NR |
| 2B: NR | 2B: NR | 2B: NR | |
| Hasan et al., [11] 2021 | 1B: 568.0±222.9 | 1B: 10.76±4.33 | 1B: 7.19±0.11 |
| 2B: 578.0±226.4 | 2B: 10.24±4.44 | 2B: 7.13±0.12 | |
| Poirier et al., [19] 2004 | 1B: 477.2±172.0 | 1B: 7.20±3.10 | 1B: 7.07±0.10 |
| 2B: 629.9±201.0 | 2B: 7.10±2.30 | 2B: 7.06±0.10 | |
| So et al., [15] 2009 | 1B: 435.2±205.8 | 1B: 10.00±5.10 | 1B: 7.19±0.13 |
| 2B: 489.5±134.4 | 2B: 10.00±4.30 | 2B: 7.17±0.10 | |
| Velasco et al., [12] 2017 | 1B: 537.3±206.7 | 1B: 10.10±4.17 | 1B: 7.20±0.11 |
| 2B: 587.8±237.1 | 2B: 10.60±4.80 | 2B: 7.13±0.10 | |
| Wolfgram et al., [13] 2022 | 1B: NR | 1B: NR | 1B: NR |
| 2B: NR | 2B: NR | 2B: NR |
Table 4.
Outcomes
| Study | Time to DKA resolution (hr), mean±SD | Time to pH resolution (hr), mean±SD | Cerebral edema, n (%) | Hypoglycemia, n (%) | Rate of CBG correction (mg/dL/hr), mean±SD | Mean rate of bicarbonate correction (mEq/L/hr), mean±SD | PICU LOS (day), mean±SD | No. of fluid bags, mean±SD | Response time for IV fluid changes (min), mean±SD | Hypokalemia, n (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Dhochak et al., [10] 2018 | 1B :20.30±14.80 | 1B: NR | 1B: 0 (0) | 1B: 6 (42.9) | 1B: NR | 1B: NR | 1B: NR | 1B: NR | 1B: NR | 1B: 9 (64) |
| 2B: 20.30±7.00 | 2B: NR | 2B: 0 (0) | 2B: 4 (26.7) | 2B: NR | 2B: NR | 2B: NR | 2B: NR | 2B: NR | 2B: 10 (67) | |
| Ferreira et al., [14] 2015 | 1B: 13.30±2.80 | 1B: NR | 1B: 0 (0) | 1B: 0 (0) | 1B: NR | 1B: NR | 1B: NR | 1B: NR | 1B: NR | 1B: 0 (0) |
| 2B: 9.80± 1.16 | 2B: NR | 2B: 0 (0) | 2B: 0 (0) | 2B: NR | 2B: NR | 2B: NR | 2B: NR | 2B: NR | 2B: 0 (0) | |
| Grimberg et al., [8] 1999 | 1B: NR | 1B: NR | 1B: 0 (0) | 1B: 0 (0) | 1B: NR | 1B: NR | 1B: NR | 1B: 8.60±1.20 | 1B: 36.70±1.40 | 1B: 0 (0) |
| 2B: NR | 2B: NR | 2B: 0 (0) | 2B: 0 (0) | 2B: NR | 2B: NR | 2B: NR | 2B: 4.80±0.30 | 2B: 7.40±1.10 | 2B: 0 (0) | |
| Hasan et al., [11] 2021 | 1B: NR | 1B: NR | 1B: 1 (1.92) | 1B: 4 (7.7) | 1B: 49.77±41.77 | 1B: NR | 1B: 1.12±0.49 | 1B: 3.29±1.21 | 1B: NR | 1B: NR |
| 2B: NR | 2B: NR | 2B: 0 (0) | 2B: 12 (21.1) | 2B: 36.93±30.23 | 2B: NR | 2B: 1.27±0.58 | 2B: 3.67±1.96 | 2B: NR | 2B: NR | |
| Poirier et al., [19] 2004 | 1B: NR | 1B: NR | 1B: 0 (0) | 1B: NR | 1B: 30.20±19.80 | 1B: 1.27±0.94 | 1B: NR | 1B: 3.20±0.42 | 1B: 2.00±30.00 | 1B: NR |
| 2B: NR | 2B: NR | 2B: 0 (0) | 2B: NR | 2B: 33.10±19.30 | 2B: 1.19±0.60 | 2B: NR | 2B: 4.10±0.60 | 2B: 1.00±1.60 | 2B: NR | |
| So et al., [15] 2009 | 1B: NR | 1B: 9.70±4.30 | 1B: NR | 1B: NR | 1B: 21.04±16.26 | 1B: 0.61±0.3 | 1B: NR | 1B: NR | 1B: NR | 1B: NR |
| 2B: NR | 2B: 9.10±7.40 | 2B: NR | 2B: NR | 2B: 31.04±20.61 | 2B: 0.95±0.55 | 2B: NR | 2B: NR | 2B: NR | 2B: NR | |
| Velasco et al., [12] 2017 | 1B: NR | 1B: 10.20±6.03 | 1B: 0 (0) | 1B: 13 (34.2) | 1B: NR | 1B: NR | 1B: 2.00±0.93 | 1B: NR | 1B: NR | 1B: NR |
| 2B: NR | 2B: 10.00±5.36 | 2B: 0 (0) | 2B: 2 (8.7) | 2B: NR | 2B: NR | 2B: 1.23±0.63 | 2B: NR | 2B: NR | 2B: NR | |
| Wolfgram et al., [13] 2022 | 1B: mean, 8.28 | 1B: NR 2B: NR | 1B: NR | 1B: 19 (20.7) | 1B: NR | 1B: NR | 1B: NR | 1B: NR | 1B: NR | 1B: NR |
| 2B: mean, 8.28 | 2B: NR | 2B: 45 (9.7) | 2B: NR | 2B: NR | 2B: NR | 2B: NR | 2B: NR | 2B: NR |
References
1. Babbitt C, Dadios M, Chau A, Tse G, Brown L, Ladbury T, et al. Implementation of an intravenous fluid titration algorithm to treat pediatric diabetic ketoacidosis. J Pediatr Intensive Care 2021;10:23–30.



2. EL-Mohandes N, Yee G, Bhutta BS, Huecker MR. Pediatric diabetic ketoacidosis. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2024 Jan-.
3. Sapru A, Gitelman SE, Bhatia S, Dubin RF, Newman TB, Flori H. Prevalence and characteristics of type 2 diabetes mellitus in 9-18 year-old children with diabetic ketoacidosis. J Pediatr Endocrinol Metab 2005;18:865–72.


4. Kamata Y, Takano K, Kishihara E, Watanabe M, Ichikawa R, Shichiri M. Distinct clinical characteristics and therapeutic modalities for diabetic ketoacidosis in type 1 and type 2 diabetes mellitus. J Diabetes Complications 2017;31:468–72.


5. Dunger DB, Sperling MA, Acerini CL, Bohn DJ, Daneman D, Danne TP, et al. ESPE/LWPES consensus statement on diabetic ketoacidosis in children and adolescents. Arch Dis Child 2004;89:188–94.



6. Glaser N, Kuppermann N. The evaluation and management of children with diabetic ketoacidosis in the emergency department. Pediatr Emerg Care 2004;20:477. –81. quiz 482-4.


7. Jayashree M, Williams V, Iyer R. Fluid therapy for pediatric patients with diabetic ketoacidosis: current perspectives. Diabetes Metab Syndr Obes 2019;12:2355–61.


8. Grimberg A, Cerri RW, Satin-Smith M, Cohen P. The "two bag system" for variable intravenous dextrose and fluid administration: benefits in diabetic ketoacidosis management. J Pediatr 1999;134:376–8.


9. Haas NL, Gianchandani RY, Gunnerson KJ, Bassin BS, Ganti A, Hapner C, et al. The two-bag method for treatment of diabetic ketoacidosis in adults. J Emerg Med 2018;54:593–9.


10. Dhochak N, Jayashree M, Singhi S. A randomized controlled trial of one bag vs. two bag system of fluid delivery in children with diabetic ketoacidosis: experience from a developing country. J Crit Care 2018;43:340–5.


11. Hasan RA, Hamid K, Dubre D, Nolan B, Sharman M. The two-bag system for intravenous fluid management of children with diabetic ketoacidosis: experience from a community-based hospital. Glob Pediatr Health 2021;8:2333794X21991532.




12. Velasco Md JP, Fogel PhD J, Levine Md PhD RL, Ciminera Md P, Fagan Md D, Bargman Md R. Potential clinical benefits of a two-bag system for fluid management in pediatric intensive care unit patients with diabetic ketoacidosis. Pediatr Endocrinol Diabetes Metab 2017;23:6–13.


13. Wolfgram PM, Frenkel M, Gage P, Sprague R, Servi A, Liggett J, et al. Standardized hospital management of pediatric diabetic ketoacidosis reduces frequency of low blood glucose episodes. Pediatr Diabetes 2022;23:55–63.



14. Ferreira JP, Penazzi M, Taborda M, Funes S, Villareal M. [A comparison of two systems for hydration of children with diabetic ketoacidosis. a randomized controlled trial]. Rev Fac Cien Med Univ Nac Cordoba 2015;72:93–9.

15. So TY, Grunewalder E. Evaluation of the two-bag system for fluid management in pediatric patients with diabetic ketoacidosis. J Pediatr Pharmacol Ther 2009;14:100–5.




16. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097.



17. Hardin AP, Hackell JM, Committee On P, Ambulatory M. Age limit of pediatrics. Pediatrics 2017;140:e20172151.



18. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5.



19. Poirier MP, Greer D, Satin-Smith M. A prospective study of the "two-bag system'' in diabetic ketoacidosis management. Clin Pediatr (Phila) 2004;43:809–13.



20. Abraham MB, Karges B, Dovc K, Naranjo D, Arbelaez AM, Mbogo J, et al. ISPAD Clinical Practice Consensus Guidelines 2022: assessment and management of hypoglycemia in children and adolescents with diabetes. Pediatr Diabetes 2022;23:1322–40.


21. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60.



22. Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol 2001;54:1046–55.

23. Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. London: The Cochrane Collaboration, 2008.
24. Nakhleh A, Shehadeh N. Hypoglycemia in diabetes: An update on pathophysiology, treatment, and prevention. World J Diabetes 2021;12:2036–49.








 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


